Catalytic reactions for C–H functionalizations are very commonly used in organic synthesis. For example, arene C−H borylations and C−H silylations can be employed. Catalytic arene C−H functionalizations that install more electropositive main group elements are less well-developed.
Stuart A. Macgregor, Heriot-Watt University, Edinburgh, UK, and University of St Andrews, UK, Michael J. Ingleson, University of Edinburgh, UK, and colleagues have developed a method for the transition-metal-free catalytic C–H zincation or C–H alumination of heteroarenes using β-diketiminate metal complexes (general reactions pictured below). Almost all C–H metalation routes to form Al/Zn organometallics require stoichiometric, strong Brønsted bases, but the team’s new reaction requires only sub-stoichiometric amounts of an amine/ammonium salt (Et3N/Et3NH+).
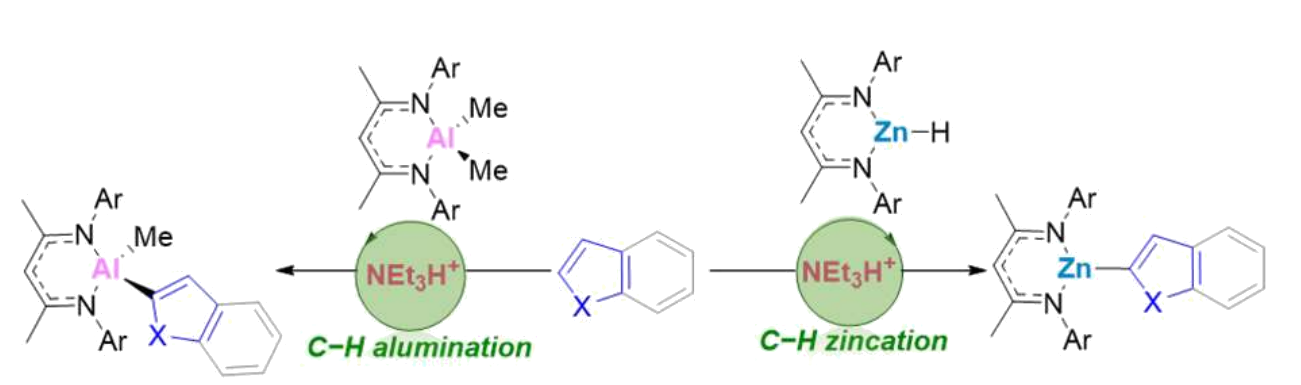
They reacted NacNacZnH or NacNacAlMe2 (NacNac = {(2,6-iPr2C6H3)N(CH3)C}2CH) with different heteroarenes such as thiophenes, furans, benzothiophenes, pyrroles, or indoles in the presence of either [Et3NH][B(ArCF3)4] (B(ArCF3)4 = B{3,5-C6H3(CF3)2}4) or [Et3NH][B(C6F5)4].
The desired products were obtained in moderate to excellent yields. The approach couples the endergonic C–H metalation with an exergonic dehydrocoupling. The only byproducts are H2 or CH4. According to the team, the approach might be transferable to other main group metals and ligand sets.
- Transition Metal‐Free Catalytic C−H Zincation and Alumination,
Milan Kumar Bisai, Justyna Losiewicz, Lia Sotorrios, Gary Nichol, Andrew Dominey, Michael Cowley, Stephen Thomas, Stuart Macgregor, Michael Ingleson,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202404848