Enzymatic electrosynthesis combines enzymatic catalysis and electrochemical techniques to obtain useful products. The technique is usually limited to oxidoreductases, with limitations in the product scope and complexity governed by the natural activity of the enzymes. Enzymes with “non-natural” catalytic activity, or enzyme promiscuity, could expand the scope of enzyme-catalyzed organic electrosynthesis.
Zhi Guan, Southwest University, Chongqing, China, and colleagues have developed an asymmetric catalysis method that combines the non-natural catalytic activity of lipase with organic electrosynthesis. The team oxidatively cross-coupled a range of 2-substituted indoles and ketones under electrochemical conditions (pictured below).
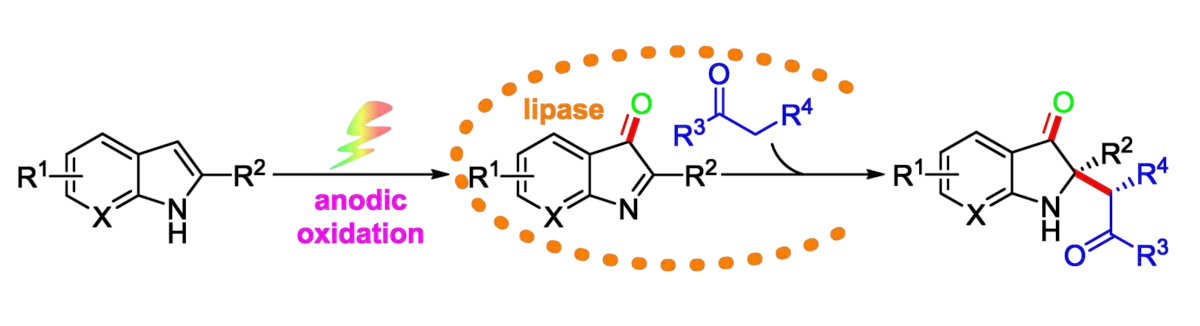
They first oxidized a 2-substituted indole to form the corresponding 2-substituted indol-3-one intermediate, using platinum sheets as electrodes, n–Bu4NPF6 as an electrolyte, and a 50:1 mixture of N,N-dimethylformamide (DMF) and water as the solvent in the presence of 4-acetamido-2,2,6,6-tetramethyl-1-piperidineoxyl (ACT). This oxidation was directly followed by an enzyme-catalyzed asymmetric alkylation with a simple aliphatic ketone using wheat germ lipase (WGL).
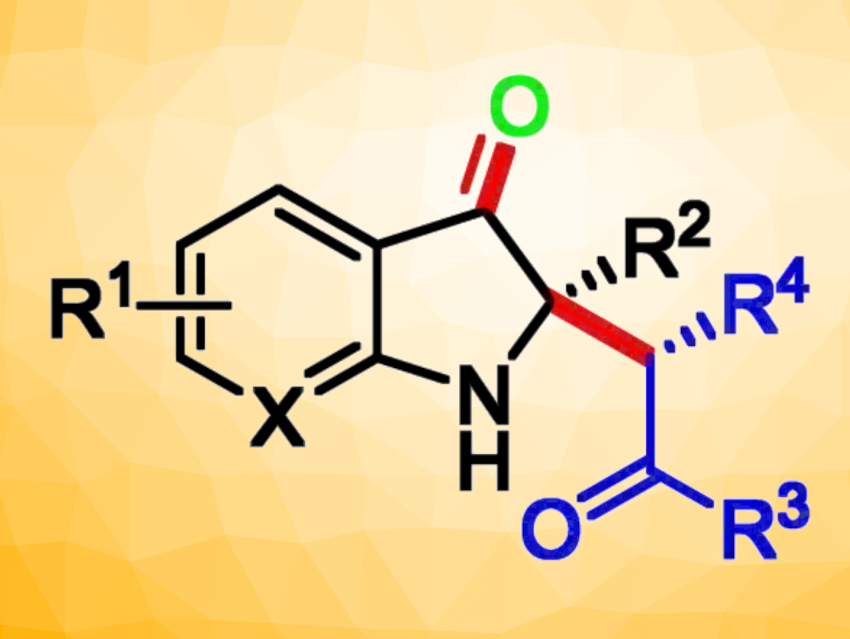
Using this approach, a series of 2,2-disubstituted indol-3-ones containing a stereogenic quaternary carbon center were prepared in good yields and enantioselectivities. According to the researchers, this is the first example of an asymmetric oxidative cross-coupling strategy that combines the non–natural catalytic activity of lipase with organic electrosynthesis.
- Merging the Non‐Natural Catalytic Activity of Lipase and Electrosynthesis: Asymmetric Oxidative Cross‐Coupling of Secondary Amines with Ketones,
Chao-Jiu Long, Huan Cao, Ben-Kun Zhao, Yu-Fang Tan, Yan-Hong He, Chu-Sheng Huang, Zhi Guan,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202203666