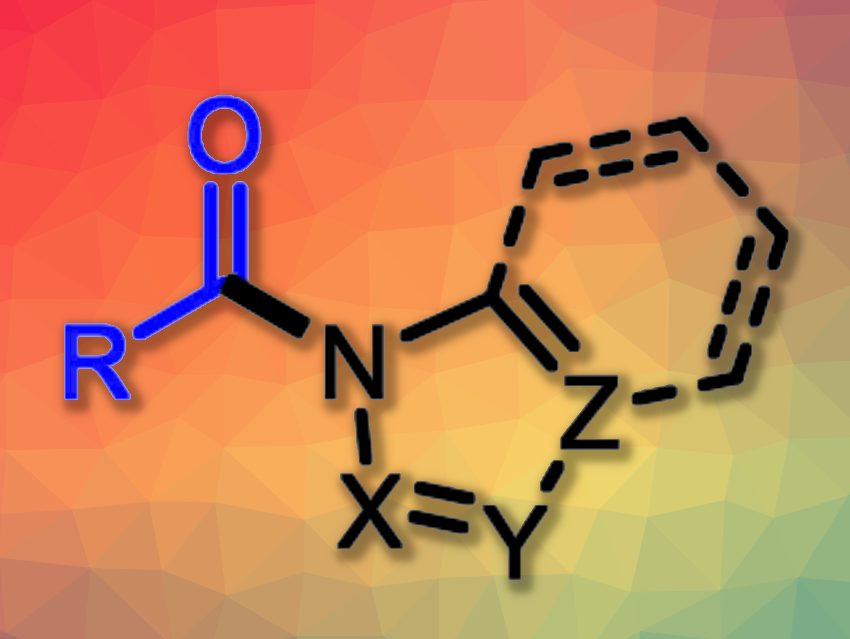
N-Acylazoles are often found as structural motifs in natural products and bioactive compounds. They can also serve as useful intermediates in organic synthesis. Usually, N-acylazoles are prepared from azoles and either carboxylic acids, anhydrides, or acid chlorides. Alternative methods that use inexpensive, easily available aldehydes instead can be useful, but usually require transition metal catalysts, strong oxidants, and/or high reaction temperatures.
Hong-Xi Li, Soochow University, Suzhou, China, and colleagues have developed an approach to the catalyst-free photochemical N-acylation of azoles with aldehydes. The team reacted azoles such as 1H-pyrazole derivatives as well as tetrazoles, triazoles, and benzimidazoles with a variety of substituted benzaldehydes in the presence of BrCCl3 as an inexpensive oxidant in toluene under 365 nm LED light at room temperature, using pyridine as a base.
Under these mild conditions, the desired acylated products were obtained in moderate to excellent yields. The researchers propose a reaction mechanism that involves the homolytic cleavage of BrCCl3 to give a trichloromomethyl radical and a bromine radical, which can then abstract a hydrogen atom from the aldehyde to give an acyl radical. The acyl radical can further react with the pyridine or another equivalent of BrCCl3 to form an activated intermediate (either an acyl bromide or an N-acyl pyridinium ion), which then reacts with the azole to give the desired product.
- Catalyst‐Free Photooxidative N‐Acylation of Azoles with Aldehydes,
Nianfeng Gong, Zelin Zhao, David James Young, Xiangqian Cao, Zhi-Gang Ren, Hong-Xi Li,
Chem. Eur. J. 2025.
https://doi.org/10.1002/chem.202404225