Diazo-diazo coupling reactions are useful tools in organic synthesis. These reactions often use metal catalysts. α-Diazo carbonyl compounds can rearrange into ketenes in a Wolff rearrangement. This rearrangement can be a useful intermediate step for the development of new diazo-diazo cross-coupling reactions.
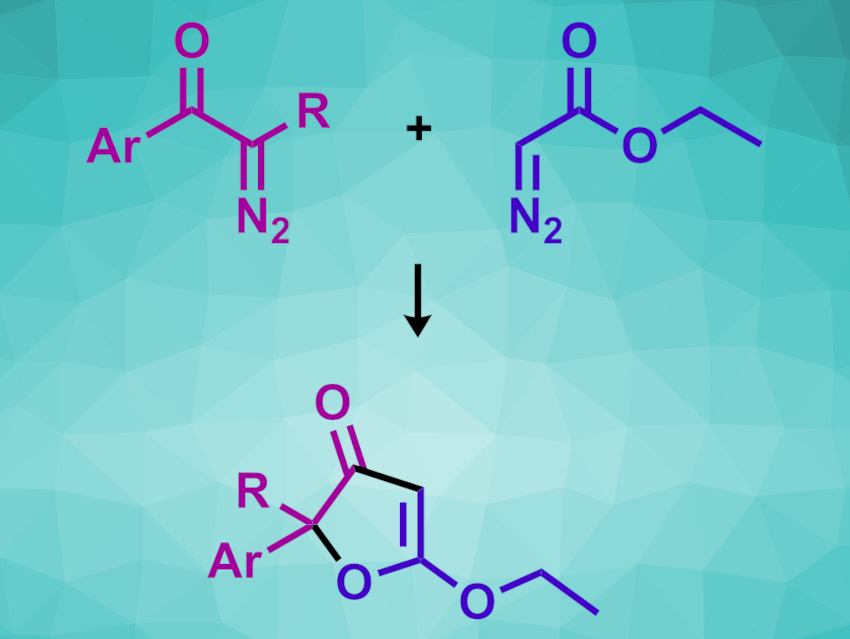
Amit Vijay Sasane and Rai-Shung Liu, National Tsing Hua University, Hsinchu, Taiwan, ROC, have developed a metal-free cross-coupling of α-aryldiazo ketones and α-diazo esters that gives 3(2H)-furanone derivatives (example pictured). The team reacted various α-aryldiazo ketones with different α-diazo esters (or ketones) at 60 °C using tetrahydrofuran (THF) as the solvent.
The desired 3(2H)-furanone derivatives were obtained in mostly good yields. The team proposes a reaction mechanism in which the α-aryldiazo ketones initially rearrange to give ketenes, which are then trapped by the second diazo species and form cyclopropanone intermediates. This is followed by cyclopropanone cleavage and an oxa-Nazarov cyclization. The prepared furanone derivatives are useful building blocks for the synthesis of, e.g., natural products.
- Catalyst-Free Diazo Cross-coupling to Access Useful 3(2H)-Furanone Derivatives,
Amit Vijay Sasane, Rai-Shung Liu,
Chem. Commun. 2022.
https://doi.org/10.1039/D2CC05912F