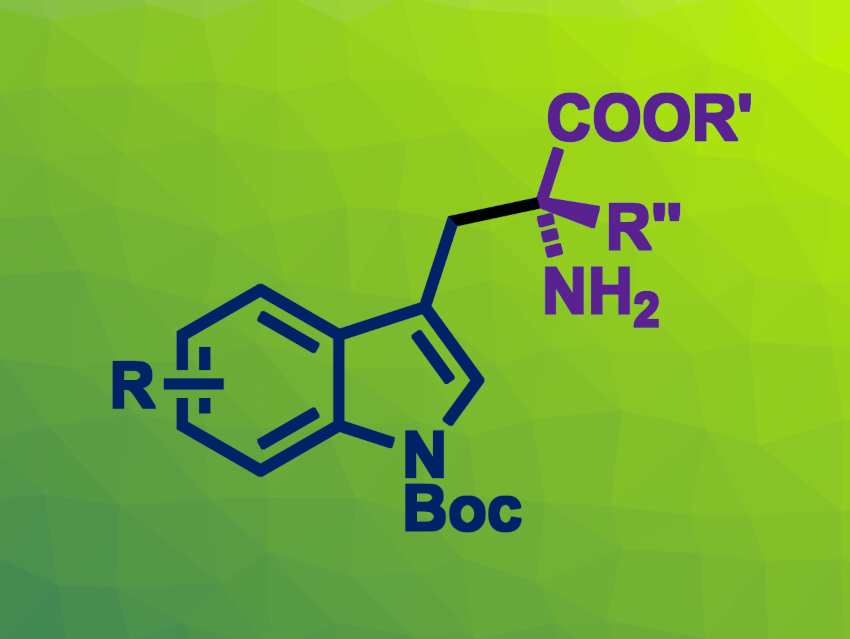
Combining organocatalysis and transition-metal catalysis can be a useful approach in organic synthesis. For example, chiral aldehyde catalysis and palladium catalysis could be used together to perform cascade reactions. Tryptophan derivatives can be useful intermediates, e.g., for the synthesis of biologically active compounds. They can be prepared. for example, from N-(2-iodophenyl)allenamide derivatives using a palladium catalyst. However, asymmetric variants of this type of reaction are less well explored.
Qi-Xiang Guo, Southwest University, Chongqing, China, and colleagues have developed an approach for a catalytic asymmetric cascade Heck-alkylation reaction of amino acid esters with N-(2-iodophenyl)allenamide derivatives to obtain optically active α-alkyl tryptophan derivatives (general product structure pictured). The team used a catalytic system that combines a chiral aldehyde, a chiral palladium complex, and ZnCl2 as a Lewis acid. They employed chiral aldehydes based on a binaphthol structure, with Pd(PPh3)4 as the palladium catalyst together with a chiral bis(diphenylphosphino)pentane ligand. The reactions were performed at 60 °C in the presence of ZnCl2 and tetramethylguanidine (TMG), using toluene as the solvent.
The desired α-alkyl tryptophan derivatives were obtained in moderate to good yields and with high enantioselectivities. The products can be further transformed without a loss in enantioselectivity. According to the researchers, the work is the first example of a chiral aldehyde/palladium-catalyzed asymmetric cascade Heck-alkylation reaction.
- Chiral Aldehyde–Palladium Catalysis Enables Asymmetric Synthesis of α-Alkyl Tryptophans via Cascade Heck-Alkylation Reaction,
Qi-Wen Shen, Wei Wen, Qi-Xiang Guo,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c01119