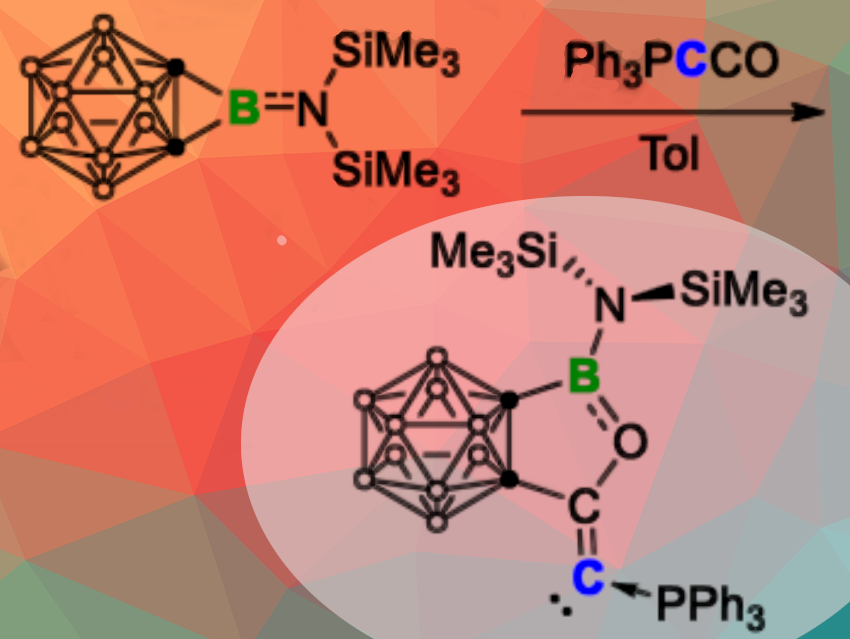
Qing Ye, Julius-Maximilians-Universität Würzburg, Germany, and colleagues have synthesized an ortho-carboranyl carbophosphinocarbene (CPC) through a click-type reaction between the super strained carborane-fused borirane 1,2-BN(SiMe3)2-1,2-C2B10H10 and the Bestmann ylide (Ketenylidenetriphenylphosphoran) Ph3PCCO (pictured above).
The researchersy then synthesized iridium (I) and gallium complexes of CPC to assess its electronic properties. With the [Cl(CO)₂Ir-CPC] and [Cl₃Ga-CPC] complexes the team confirmed CPC’s exceptional electron-donating ability through Tolman Electronic Parameter (TEP) and Cl–Ga–Cl bond angles.
Preliminary reactivity investigations demonstrate that the new CPC readily forms adducts with boranes and can effectively stabilize borenium cations. Its strong nucleophilicity enables reactions with carbon dioxide, while its exceptional Brønsted basicity allows it to deprotonate imidazolium to generate carbene species.
The researchers plan to further explore super-strained C₂B-boracycles as click reagents for the synthesis of boron-containing functional molecules and ligands, as well as systematic studies on carboranyl CPC, are currently underway, the researchers say.
- Conversion of Bestmann Ylide into Carbophosphinocarbene,
Libo Xiang, Junyi Wang, Niclas Knoblauch, Alexander Matler, Qing Ye,
Angew. Chem. Int. Ed. 2025.
https://doi.org/10.1002/anie.202501955