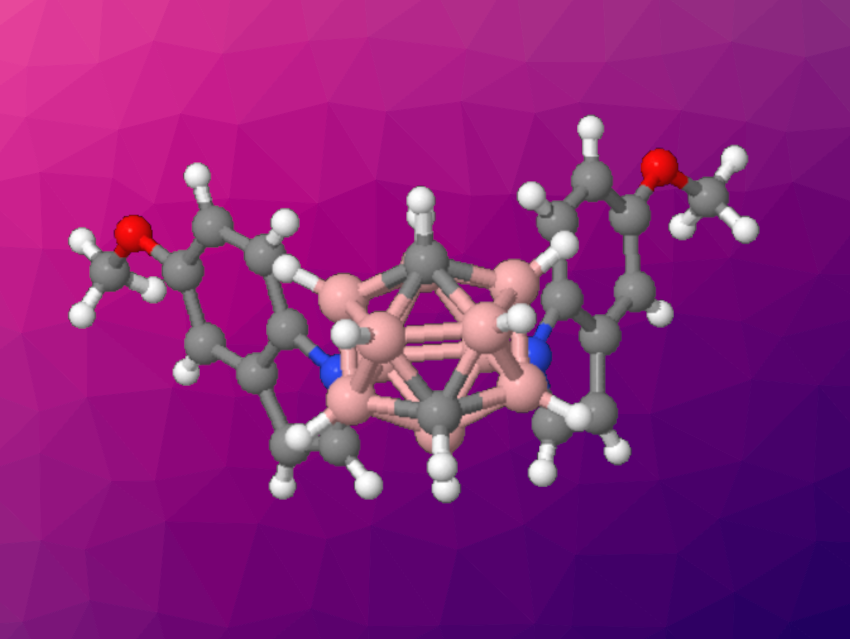
Carboranes are clusters composed of boron, carbon, and hydrogen atoms. They have applications, e.g., in materials chemistry, and methods for their functionalization are interesting research targets. Installing nitrogen-containing units at the boron vertices, for example, can lead to potentially useful structures.
Alexander M. Spokoyny, University of California, Los Angeles, USA, Chao Zou, Songshan Lake Materials Laboratory, Dongguan, Guangdong, China, Xin Mu, East China University of Science and Technology, Shanghai, and colleagues have developed a Pd-catalyzed process to connect different NH-heterocycles, anilines, or heteroanilines to vicinal boron vertices of a carborane. The team started from 9,10-Br2–meta-carborane, which was used in double cross-coupling reactions with, e.g., indoles, carbazoles, pyrroles, anilines, heteroanailines, etc., employing Pd(OAc)2 as a catalyst together with a triarylphosphine ligand with three ortho-methoxyl groups in the presence of K3PO4. The reactions were performed at 100 °C.
The team obtained the desired disubstituted carboranes in mostly moderate to good yields. Sequential couplings were also possible, giving unsymetrically disubstituted products. In addition, the researchers found that oxidative cyclizations of the products obtained using indoles and pyrroles led to a new class of six- and seven-membered heterocycles fused to carboranes via two boron atoms.
- A Pd-Catalyzed Route to Carborane-Fused Boron Heterocycles,
Mengjie Zhu, Puzhao Wang, Zhengqiu Wu, Yangfa Zhong, Laiman Su, Yuquan Xin, Alex Spokoyny, Chao Zou, xin mu,
Chem. Sci. 2024.
https://doi.org/10.1039/D4SC02214A