Plastics are inescapable in our daily lives. The vast amounts of plastic garbage heaped in landfills and in the environment, however, are as problematic as the plastics are useful. Lutz Ackermann, Wöhler Research Institute for Sustainable Chemistry, Göttingen, Germany, and colleagues have developed a new method for recycling polystyrene (PS) waste. The team’s efficient electrochemical process uses an inexpensive iron catalyst, produces hydrogen as a byproduct, and can be powered by solar panels.
A Pile of Plastic Waste
Less than 10 % of the plastic produced in the world is recycled. Plastic waste is accumulating in landfills and waterways, threatening wildlife and the environment. By 2025, this “pile” of plastic is predicted to reach 40 billion tons. Globally, about 33 % of the material deposited in landfills consists of polystyrene, which is widely used in packaging and construction. Only about 1 % of polystyrene is recycled.
The worldwide production capacity of polystyrene reached 15.4 million tons in 2022 and continues to increase. Recycling of plastics, particularly polystyrene, is one of the biggest societal challenges of our time. Efficient, cost-effective recycling methods that convert plastic waste to valuable small molecules that can be used in chemical syntheses would be a step toward a sustainable circular carbon economy.
Efficient Electrochemical Recycling
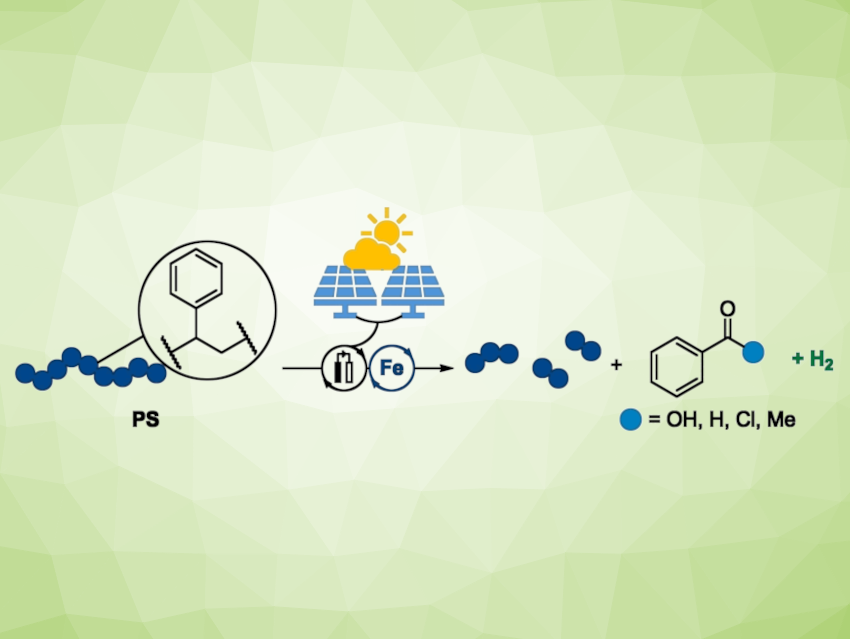
The team developed an electrocatalytic method for the efficient degradation of polystyrenes. The degradation produces a relatively high fraction of monomeric benzoyl products that can be used as starting materials for chemical processes, as well as some short polymer chains.
The key to this success is a powerful iron-based catalyst, an iron porphyrin complex that resembles hemoglobin. Its advantage over many other catalytically active metals is that iron is nontoxic, inexpensive, and easy to obtain. During the electrocatalytic reaction, the iron compound cycles between different oxidation steps (IV, III, and II).
Monomeric Benzoyl Products and Hydrogen Evolution
The developed iron-catalyzed degradation results in the splitting of the carbon–carbon bonds in the polymer backbone. The main products are benzoic acid and benzaldehyde. Benzoic acid is a starting material for a variety of chemical syntheses in the production of scents and preservatives, for example. The robustness of this novel electrocatalysis method was demonstrated by the efficient degradation of real-life plastic waste on the gram scale.
This polystyrene degradation process could be fully powered with electricity from commercially available solar panels. In addition, a useful side reaction occurs during the degradation process: the production of hydrogen. In this way, the new electrocatalytic process, which can easily be scaled to an industrial level, combines efficient plastic recycling with decentralized, green hydrogen production.
- Anodic Commodity Polymer Recycling: The Merger of Iron‐Electrocatalysis with Scalable Hydrogen Evolution Reaction,
Maxime Hourtoule, Sven Trienes, Lutz Ackermann,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202412689


I am really happy to read such articles, I can learn many new information in chemistry.
i loove this journal