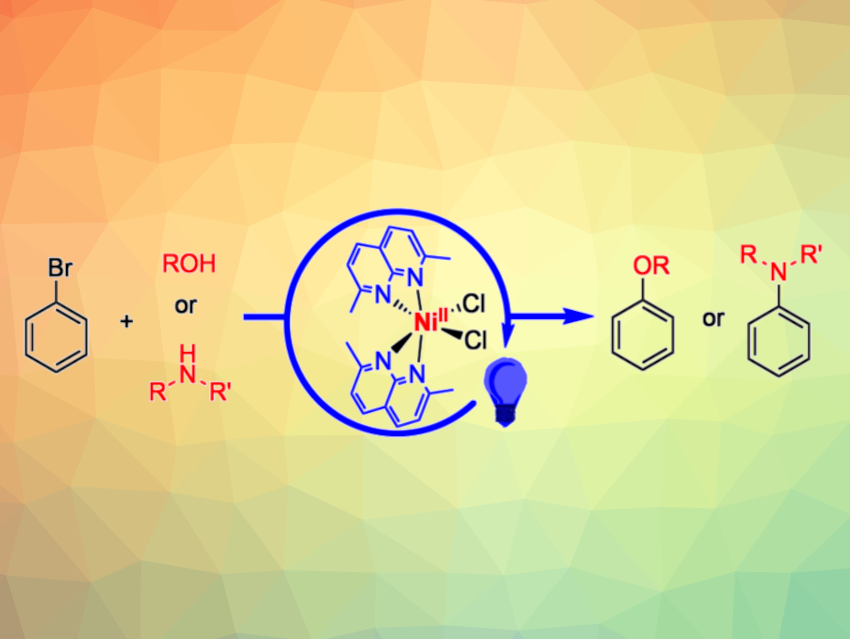
The development of catalysts based on Earth-abundant, inexpensive metals—combined with energy provided by light—for the construction of C-N and C-O bonds is an interesting research target. Such catalysts could provide economical and sustainable pathways for the synthesis of amines and alcohols, which serve as important commodity chemicals.
Julia R. Khusnutdinova, Okinawa Institute of Science and Technology Graduate University, Japan, and colleagues have found that an air-stable nickel(II) chloro complex supported by a 2,7-dimethyl-1,8-naphthyridine ligand can act as a universal catalyst for both C–O and C–N cross-coupling reactions. The catalyst promotes reactions of aryl bromides with amines or alcohols under blue or purple LED light (simplified reactions pictured), without requiring a precious metal photosensitizer. The nickel complex was prepared by reacting the ligand with the Ni dichloride precursor (PMe3)2NiCl2 at 60 °C for 2 d in methanol.
For C–N cross-coupling reactions, the team used the developed catalyst together with blue LED light and dimethylacetamide (DMA) as the solvent. For C–O cross-coupling reactions, they employed purple LED light and used the alcohol coupling partner as the solvent in the presence of tri-n-propylamine as a base. The desired coupling products were obtained in moderate to excellent yields, depending on the substrates. The work shows that dimethylnaphthyridine could serve as a versatile and convenient ligand scaffold to explore photocatalytic applications of first-row transition metals.
- Photoinduced Carbon‐Heteroatom Cross‐Coupling Catalyzed by Nickel Naphthyridine Complexes,
Janet Bahri, Shubham Deolka, Pavan K. Vardhanapu, Eugene Khaskin, Ramadoss Govindarajan, Robert R. Fayzullin, Serhii Vasylevskyi, Julia Khusnutdinova,
ChemCatChem 2023.
https://doi.org/10.1002/cctc.202301142