Methods for the functionalization of unactivated C–H bonds are useful in organic synthesis. One approach to this type of reaction can be the insertion of carbenes into the bond in question. However, it can be challenging to achieve the necessary regioselectivity in intermolecular variants of this kind of transformation.
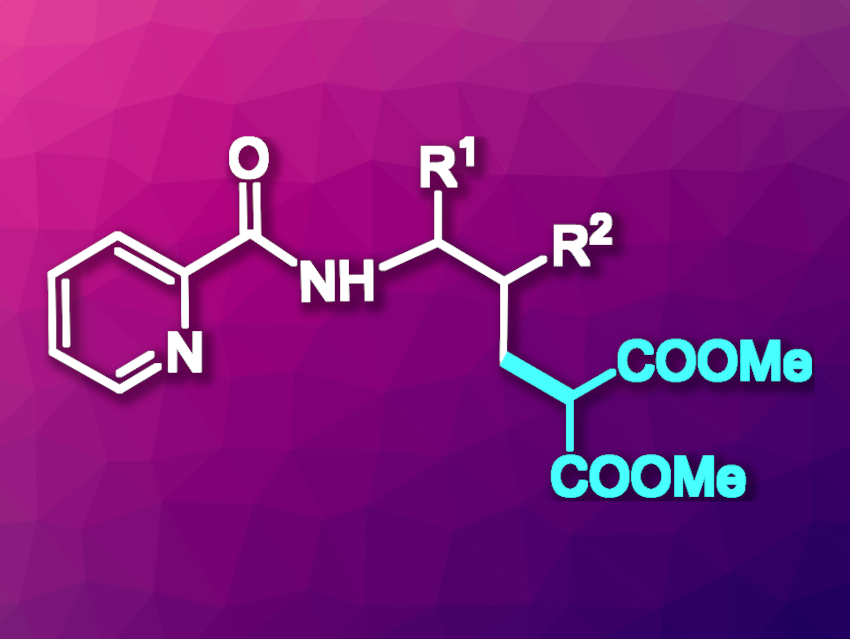
Ming Yan, Sun Yat-Sen University, Guangzhou, China, and colleagues have developed a method for the regioselective insertion of carbenes into the γ-C(sp3)–H bonds of aliphatic amines (general product structure pictured). The team reacted derivatives of N-isobutylpicolinamide with dimethyl diazomalonate, which acts as a carbene precursor. They used Pd(OAc)2 as a catalyst, dibenzylideneacetone (dba) as a ligand, potassium pivalate (KOPiv) as a base, LiF as an additive, and 1,2-dichloroethane (DCE) as the solvent. The reactions were performed at 120 °C under a nitrogen atmosphere.
Under these conditions, the desired insertion products were obtained in moderate to good yields. According to the researchers, the picolinamide unit acts as a directing group, which ensures a high regioselectivity for the key step, a palladium-catalyzed γ-C(sp3)–H activation.
- Palladium-Catalyzed Regioselective Insertion of Carbenes into γ-C(sp3)–H Bonds of Aliphatic Amines,
Peng Zhang, Cheng-Xin Li, ShihaoZhi Wang, Xue-Jing Zhang, Ming Yan,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c00038