Reducing nitroarenes is important for both industry and the environment. Since these compounds are useful but harmful when released, finding cleaner and safer ways to convert them is key to sustainable chemical production.
Luísa Margarida Martins, Universidade de Lisboa, Portugal, discusses a recently published study in which she and her colleagues explore the effects of catalysts, solvents, and reducing agents on the reaction kinetics of nitroarene reduction.
What did you do?

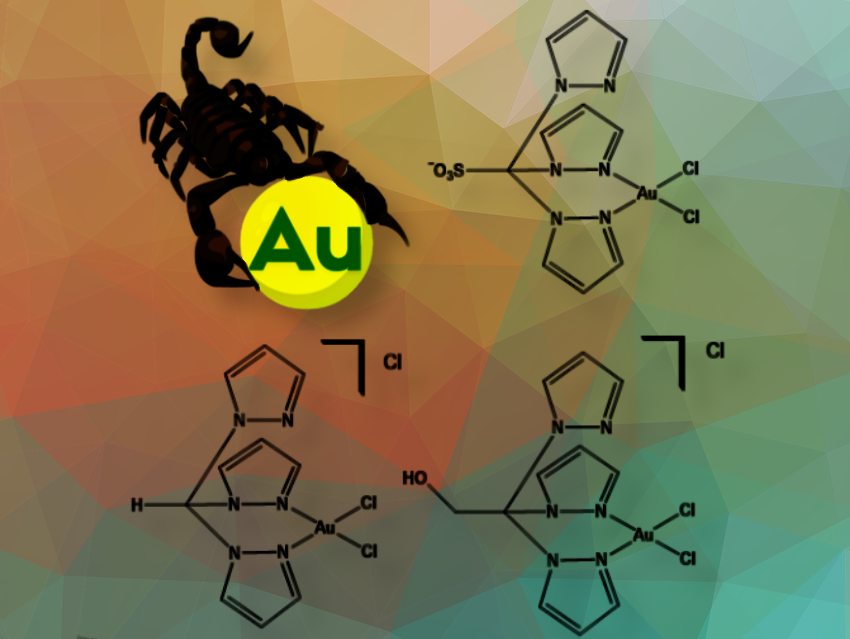
We developed an efficient catalytic system for the rapid and selective reduction of nitroarenes to aminoarenes under mild conditions. Our approach uses environmentally friendly, water-prepared C-scorpionate gold(III) complexes, eliminating the need for harsh reagents or extreme reaction conditions.
Why are you doing this?
Nitroarenes are key intermediates in various industries, including pharmaceuticals, petrochemicals, agriculture, and dyes. However, their accumulation in the environment poses risks to ecosystems and human health. Conventional reduction methods rely on platinum- or palladium-based catalysts, often requiring harsh conditions or toxic reagents. Our goal was to develop a more efficient, selective, and environmentally friendly method for converting nitroarenes into biodegradable compounds, such as aminoarenes.
What is new and cool about your approach?
This was the first time C-scorpionate gold(III) complexes were applied in nitroarene reduction. An improved catalytic system with broad applicability was developed, achieving higher rate constants compared to gold nanoparticles and other metal complexes found in the literature.
Moreover, our new catalytic system offers remarkable selectivity, enabling targeted reduction of nitro groups while preserving other functional groups—a key advantage for complex molecule synthesis. We were able to operate under mild reaction conditions, which could make the process more sustainable and cost-effective.
What are your key findings?
Our key findings demonstrate that the reaction selectivity is tuneable, depending on the catalyst structure and reaction conditions.
Moreover, the choice of solvent and reducing agent significantly impa cts catalytic performance, allowing for further optimization. These results contribute to the broader field of sustainable catalysis, offering practical insights for green chemistry applications.
cts catalytic performance, allowing for further optimization. These results contribute to the broader field of sustainable catalysis, offering practical insights for green chemistry applications.
What is the longer-term vision for your research?
Our findings could directly impact optimizing catalyst design and advancing green chemistry by reducing reliance on toxic reagents and harsh conditions. In the long term, our research may contribute to commercially viable gold-based catalysts for pharmaceutical synthesis, as well as for the production of other fine chemicals and agrochemicals.
What part of your work was the most challenging?
One of the main challenges was optimizing the catalytic reaction parameters to balance efficiency, selectivity, and sustainability. Achieving high conversion rates while maintaining functional group tolerance required extensive experimentation and fine-tuning of the reaction conditions.
Anything else you would like to add?
This work underscores the growing potential of C-scorpionate complexes in catalysis and highlights the importance of developing sustainable chemical processes. We hope our findings will inspire further exploration of gold-based catalysts in green chemistry applications.
Thank you very much for sharing these insights.
The paper they talked about:
- C-Scorpionate Gold(III) Catalysts in Nitroarene Reduction: A Novel Strategy,
Hugo Lapa, Vanmira Van-Dúnem, Vânia André, Wojciech Gil, Elisabete Alegria, Anna Trzeciak, Luisa Martins,
ChemCatChem 2025.
https://doi.org/10.1002/cctc.202500251

Luísa Margarida Martins is Full Professor of the Chemical Engineering Department of Instituto Superior Técnico, Universidade de Lisboa, Portugal