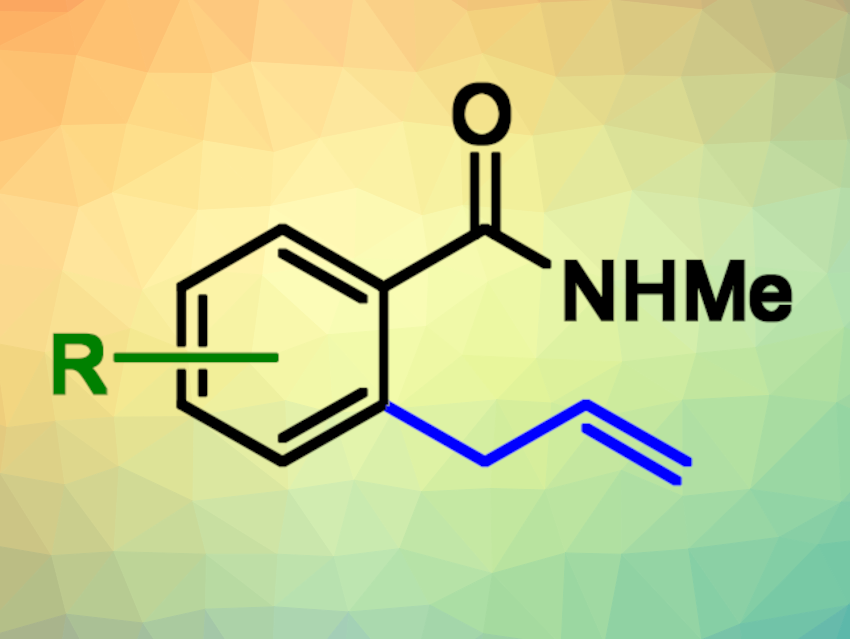
Allyl arenes are common structural motifs in natural products and bioactive compounds, for example, in compounds that are important in the flavor and fragrance industry. In addition, the allyl group is a versatile functional group for further functionalizations. The transition-metal-catalyzed C(sp2)−H allylation of aromatic substrates can be an attractive route to allylated arenes in terms of atom- and step-economy, functional group compatibility, and control of regioselectivity.
Nuria Sotomayor, Esther Lete, Universidad del País Vasco / Euskal Herriko Unibertsitatea (UPV/EHU), Bilbao, Spain, Enrique Gómez Bengoa, UPV/EHU, San Sebastián, Spain, and colleagues have found that allyl aryl ethers can be used for the C(sp2)-H allylation of (hetero)arenes using Earth-abundant cobalt catalysts (pictured below), using an amide as a directing group. The team reacted a series of aromatic amides with different allyl aryl ethers, using Cp*CoI2(CO) as the catalyst in the presence of AgSbF6 and PhCO2K with 1,2-dichloroethane (DCE) as the solvent. The reactions were performed at 80 °C.
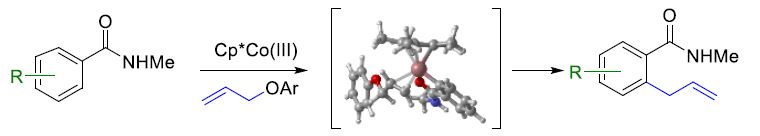
The researchers used density functional theory (DFT) calculations to investigate the reaction. They found that in most cases, steric effects play a less significant role than electronic effects in the reactivity of the substituted aromatic reactants. The reaction can be applied to a variety of substituted (hetero)aromatic compounds, and the allylated products could be easily transformed further.
- Directed C–H Allylation of Aromatic Carboxamides with Allyl Aryl Ethers under Cp*Co(III)‐Catalysis,
Asier Carral-Menoyo, Iratxe Barbolla, Carlos Santiago, Martín Espinel, Nuria Sotomayor, Enrique Gómez-Bengoa, Esther Lete,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202301090