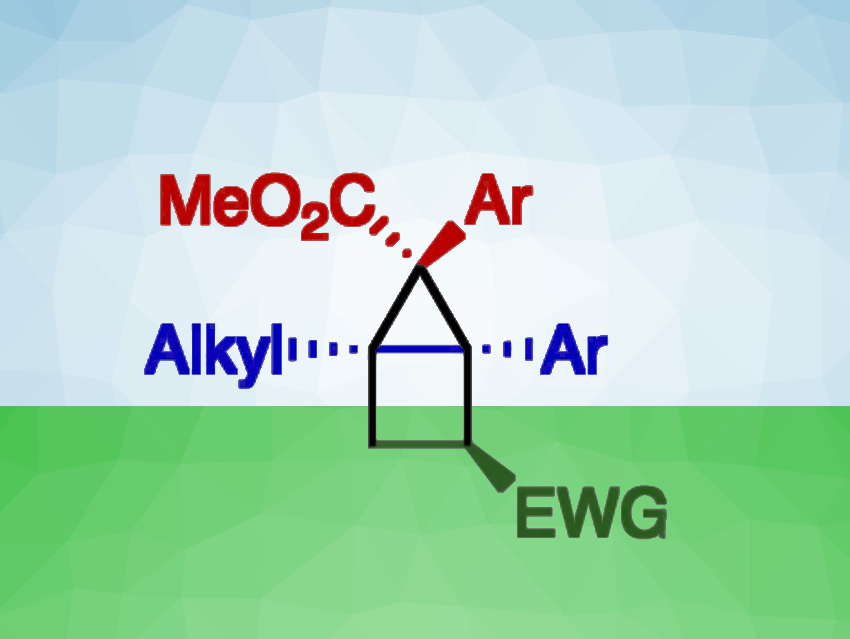
Bicyclo[2.1.0]pentanes (housanes) are bicyclic systems composed of a three-membered ring fused to a four-membered ring—i.e., they look like a simple drawing of a house. These strained molecules can be challenging to synthesize. [2+2] Cycloadditions of cyclopropenes to alkenes offer an attractive path. The cyclopropenes can, in turn, be prepared via a cyclopropenation of alkynes using aryldiazoacetates.
Geraint H. M. Davies, PostEra, Cambridge, MA, USA, Robert R. Knowles, Princeton University, NJ, USA, Huw M. L. Davies, Emory University, Atlanta, GA, USA, and colleagues have used these two steps to develop a method for the regioselective and stereoselective synthesis of highly functionalized bicyclo[2.1.0]pentanes. The team first used silver- or gold-catalyzed reactions to generate the cyclopropene building blocks in an enantioselective manner from aryldiazoacetates and alkynes under loss of N2. This was followed by a [2+2] cycloaddition step using Ir photocatalysts under blue LED light to construct the four-membered ring from the cyclopropene and an alkene.
The desired housanes were obtained with good regio- and stereocontrol. When enantioenriched cyclopropenes were used, the synthesis proceeded under enantioretention. The reactions have a comparatively broad substrate scope. Thus, the work provides stereoselective access to a range of functionalized bicyclo[2.1.0]pentanes.
- Stereoselective Synthesis of Highly Functionalized Bicyclo[2.1.0]pentanes by Sequential [2 + 1] and [2 + 2] Cycloadditions,
Brockton Keen, Christina Cong, Alberto Castanedo, Geraint H. M. Davies, Robert R. Knowles, Huw M. L. Davies,
Org. Lett. 2025.
https://doi.org/10.1021/acs.orglett.5c00054