Understanding non-covalent interactions is important, e.g., to predict crystal structures. Improved models for interactions such as van der Waals forces or halogen bonds can be useful for theoretical studies of compounds in condensed phases. Bromopentafluorobenzene (C6F5Br), is an aromatic organofluorine compound that is liquid at room temperature (melting point: –31 °C, or 242 K). It is an interesting model compound for studies of non-covalent interactions because it can form stacked co-crystals with other aromatic compounds. The single bromine atom allows for comparison with similar co-crystals featuring, for example, C6F6 to understand the effects of the substituents on the structures formed in the solid state.
As part of an investigation of non-covalent interactions in co-crystals, Jeremy K. Cockcroft, University College London, UK, and colleagues have studied the solid-state structures of bromopentafluorobenzene as a reference “pure” phase. The team used differential scanning calorimetry (DSC) to identify melting and solid-state phase transitions, as well as variable-temperature powder X-ray diffraction (VT-PXRD) to track phase and volume changes with changing temperatures, and single-crystal X-ray diffraction (SXD) at a constant temperature for structure determination of the different phases.
The researchers first found a single solid-state phase below the melting point. However, a closer look at the DSC data showed some unexpected behavior: a weak exothermic transition at 233 K, which was followed by a weak endothermic transition of a similar magnitude on heating—but the team observed no equivalent transitions upon cooling. Indeed, VT-PXRD data showed evidence of three solid-state phases obtained upon heating. Repeat measurements with smaller temperature steps upon heating revealed an additional fourth phase.
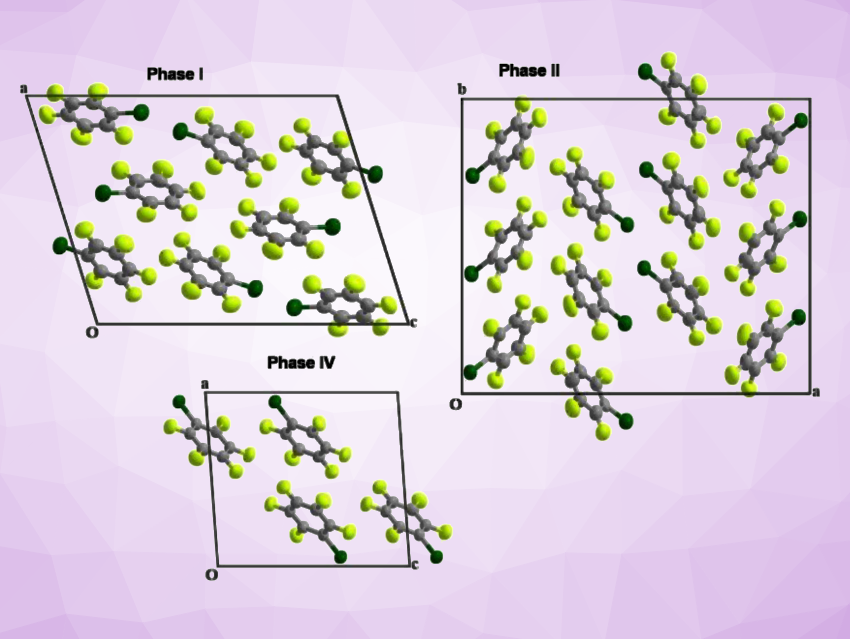
It first seemed like the lowest-temperature phase (phase IV) exists over a wide range of temperatures, while phases I, II, and III exist closer to the melt. However, the team found that kinetics play an important role in the phase behavior of bromopentafluorobenzene—phase I, for example, is kinetically stable upon cooling to low temperatures, so crystals of phase II could only be obtained via warming of phase IV and not by cooling of phase I. The researchers solved the crystal structures for phases I, II, and IV (pictured), while phase III remained elusive. Overall, the compound shows a relatively complex phase behavior, which needs to be considered, for example, in crystal structure prediction.
- The Changing Phases of Bromopentafluorobenzene,
Jeremy Karl Cockcroft, Joseph C. Bear,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202402867