Sulfur hexafluoride (SF6) is fairly inert, which makes it useful for different applications, e.g, as an electrical insulator. However, it also is a potent greenhouse gas with a very long lifetime in the atmosphere. Thus, methods for its degradation are interesting research targets—in particular, catalytic approaches.
Josep Cornella, May Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, and colleagues have found that certain pincer bismuthinidene complexes can degrade both SF6 and the related compound PhSF5. The team first reacted a symmetric N,C,N-bismuthnidene featuring two tert-butyl substituents with SF6 in the presence of lithium triflate. This led to new bismuth species, a Bi(II) dimer formed from the pincer complex and a sulfide-bridged Bi(III) dimer. Thus, the pincer complex can activate SF6. A reaction of the same pincer complex with PhSF5 gave a new bismuth(III) thiophenolate species.
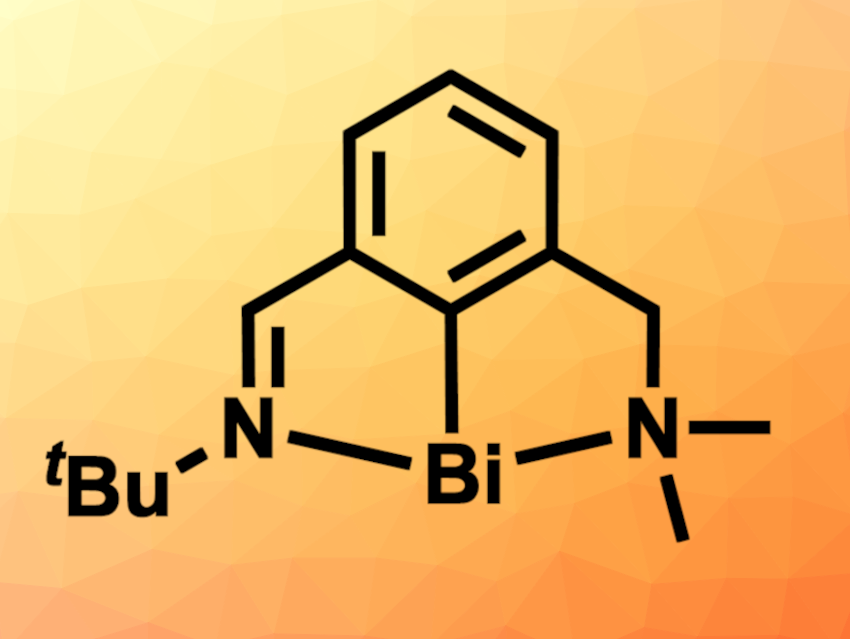
The researchers then switched to a bismuthinidene with an asymmetric pincer ligand (pictured), in which one imine unit was replaced with a dimethyl-substituted amine. This species was able to catalytically degrade both SF6 and PhSF5 in the presence of PMe3 as a reducing agent, giving S=PMe3 and F2PMe3 or PhSH and O=PMe3, respectively. According to the researchers, this represents the first example of the catalytic degradation of PhSF5.
- Activation and Catalytic Degradation of SF6 and PhSF5 at a Bismuth Center,
Vanessa A. Béland, Nils Nöthling, Markus Leutzsch, Josep Cornella,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c07044