Metal–metal bonded compounds can have interesting bonding situations and reactivities. Covalently bonded tri-metallic “chains”, for example, can feature a central metal atom with a formal oxidation state of zero—i.e., they can be described as systems of the type M(I)–M'(0)–M(I). There are, e.g., a known bis(aluminyl)cuprate and a bis(aluminyl)samarium complex.
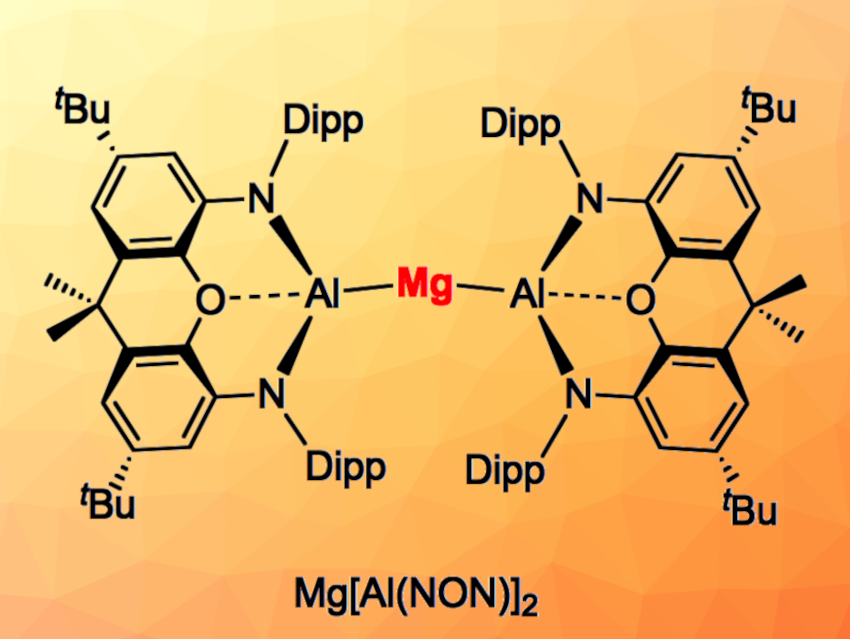
Simon Aldridge, University of Oxford, U, and colleagues have synthesized a bis(aluminyl) magnesium complex (Mg[Al(NON)]2, pictured) that shows an interesting dual reactivity. The team reacted the potassium aluminyl compound K2[Al(NON)]2 with MgI2 in toluene, followed by recrystallization from pentane to obtain the product in the form of yellow crystals in a yield of 80 %. The compound was characterized using NMR and infrared (IR) spectroscopy, elemental analysis, and X-ray diffraction. The results confirmed the formation of the desired magnesium bis(aluminyl) complex, which is a rare example of a complex with only aluminyl supporting ligands. The trimetallic chain has a bent structure with an Al–Mg–Al angle of 164.8° and comparatively short Mg–Al bonds.
Mg[Al(NON)]2 can act as a four-electron reductant, reacting with SF6 at 80 °C to give Mg[(μ-F)2Al(NON)]2. The researchers also found evidence for both radical chemistry and nucleophilic behavior at aluminium. The compound can, e.g., react with substrates such as MeI via a nucleophilic attack through aluminium. Under photolytic activation, the team observed a reaction with 1,5-cyclooctadiene that is consistent with radical-based chemistry.
- Bis(aluminyl)magnesium: a source of nucleophilic or radical aluminium‐centred reactivity,
Liam Griffin, Mathias Ellwanger, Jonathon Clark, William Myers, Aisling Roper, Andreas Heilmann, Simon Aldridge,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202405053