The ever-growing amounts of plastic waste add up to an important environmental issue. Some polymer materials can be recycled via mechanical methods, while other polymer waste is suited to chemical recycling, in which monomers or other small molecules are obtained from the polymer and then reused as a chemical feedstock. Ideally, new polymer materials should be designed with recyclability in mind and use biobased rather than fossil resources. The ring-opening polymerization of fused lactones, for example, could be used to create recyclable polyesters, but it can be challenging to find suitable biobased lactone monomers.
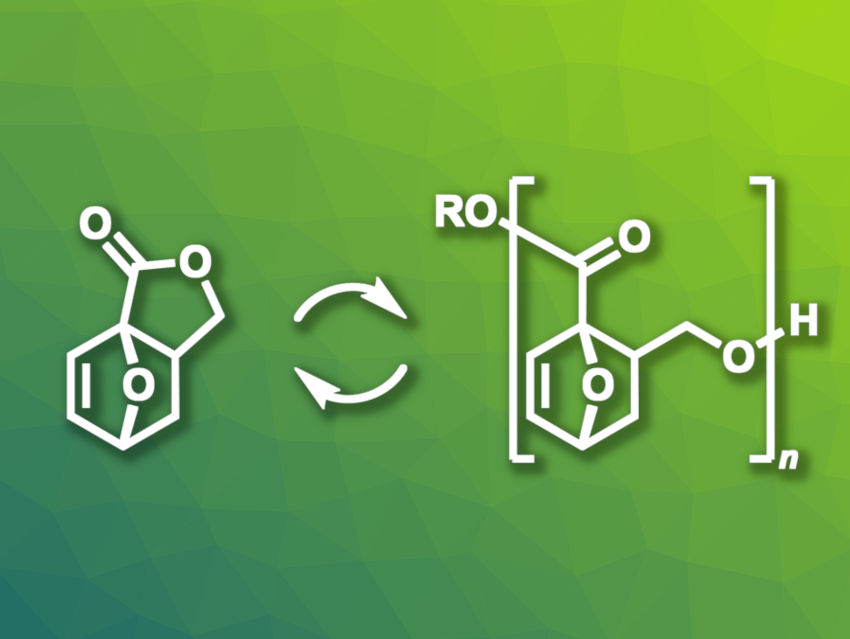
Arnaud Thevenon, Pieter C. A. Bruijnincx, Utrecht University, The Netherlands, and colleagues have used a biobased tricyclic γ-butyrolactone with an oxanorbornene unit as a monomer to obtain a chemically recyclable polyester (pictured). The monomer can be synthesized in high yields from allyl alcohol and a furfural—low-cost building blocks that can be obtained from renewable sources. The team found that this monomer underwent fast and controlled polymerization, while also allowing chemical depolymerization for recycling. The desired polyester was formed in low dispersity and with controllable molecular weights.
The poly(oxanorbornene-fused γ‑butyrolactone) formed from the tricyclic lactone monomer is suitable for further modifications after the polymerization, which gives researchers the option to tune the polymer properties. As an example, they found that the hydrogenation of the alkene group significantly increased the polymer’s thermal stability. Other derivatives of the monomer could also be used to form polymers with fine-tuned properties. Thus, the developed polymer can serve as the model for an entire family of possible new, recyclable, bio-based polymers.
- Closed-Loop Chemical Recycling of a Biobased Poly(oxanorbornene-fused γ-butyrolactone),
Eva Harsevoort, Răzvan C. Cioc, Martin Lutz, Arnaud Thevenon, Pieter C. A. Bruijnincx,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c12678
![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)


