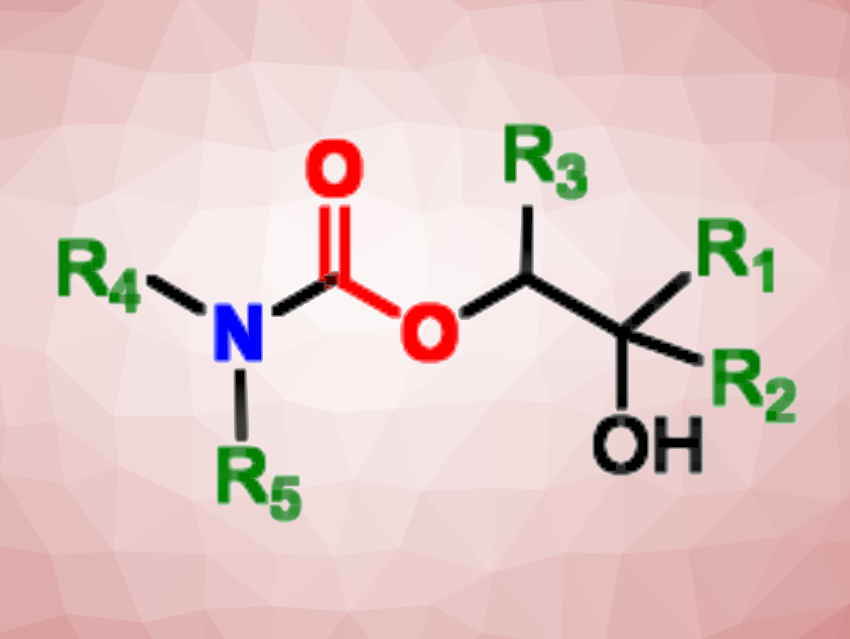
Hydroxyurethanes, or hydroxy-functionalized carbamates, are found, e.g., in biologically active compounds or agrochemical products. Their synthesis can be challenging as it can require the use of very reactive and/or toxic reagents such as carbon monoxide, phosgene, or isocyanates. Safer and more sustainable methods for their preparation are, therefore, in demand.
Felipe de la Cruz-Martínez, José A. Castro-Osma, Agustín Lara-Sánchez, Universidad de Castilla-La Mancha, Albacete, Spain, and colleagues have developed a method for the synthesis of bio-derived hydroxyurethanes from cyclic carbonates that uses solvent-free conditions and TBD (1,5,7-triazabicyclo[4.4.0]dec-5-ene) as an organocatalyst (reaction pictured below).
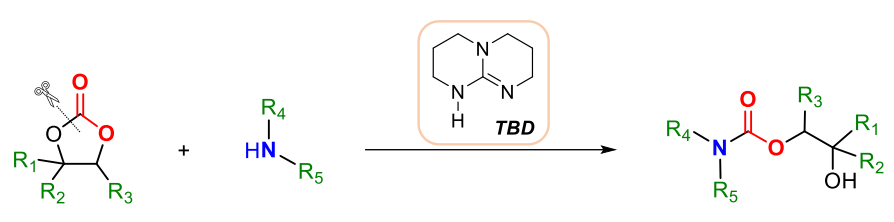
In this approach, the reactants also act as the solvents, thus reducing chemical waste. The reaction is regioselective: One specific C–O bond is cleaved due to steric hindrance around the carbonate unit. The method was applied to furan-, carvone- and limonene-based cyclic carbonates, giving a wide range of biosourced hydroxyurethanes. The products could be used to produce renewable polymers.
- Base‐catalyzed Highly Regioselective Synthesis of Bio‐based Hydroxyurethanes,
Felipe de la Cruz-Martínez, Enrique Francés-Poveda, Michael North, José Antonio Castro-Osma, Agustín Lara-Sánchez,
Eur. J.Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202400275