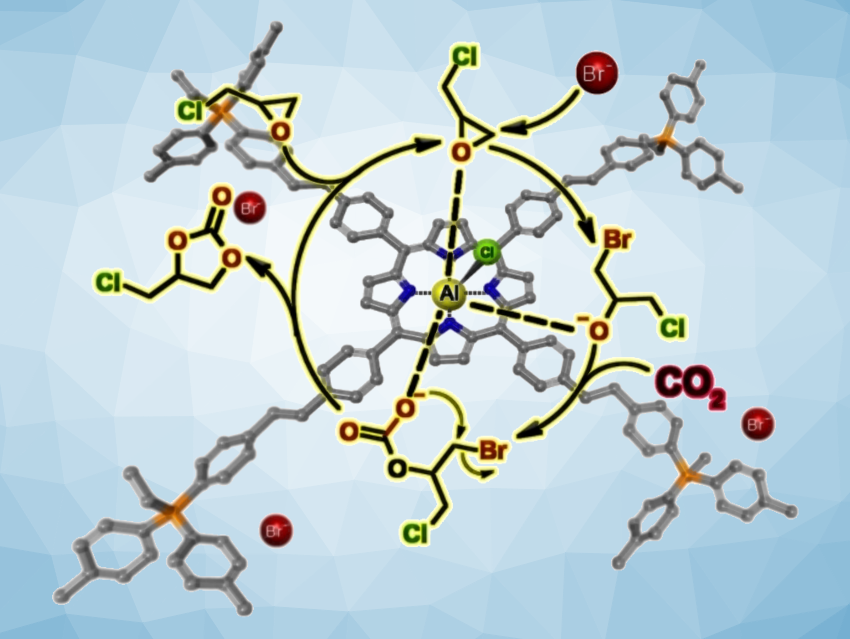
Two catalytic sites can be better than one when they synergistically operate in a chemical process. The incorporation of CO2 into epoxides to generate cyclic carbonates is interesting due to the possibility of using the greenhouse gas as a low-cost and renewable carbon feedstock. Catalytic systems that combine Lewis-acidic sites for the electrophilic activation of the epoxy substrate with nucleophilic species for promoting epoxide ring opening are interesting in this context.
Michelangelo Gruttadauria, Francesco Giacalone, University of Palermo, Italy, Carmela Aprile, University of Namur, Belgium, and colleagues have searched for reusable catalysts for the efficient conversion of CO2 and epoxides into cyclic carbonates. The team observed synergy in a catalytic material based on aluminium chloride tetrastyrylporphyrin copolymerized with a vinyl-functionalized quaternary phosphonium bromide salt. The copolymer, called TSP-AlCl-PhospBr, was obtained via a simple one-pot radical polymerization using the thermal radical initiator 2,2′-azobis(2-methylpropionitrile) (AIBN) and the monomers, i.e., the aluminium porphyrin (TSP-AlCl) and the phosphonium salt.
According to the researchers, the close proximity of the Lewis acid site and the bromide anions leads to a much higher catalytic efficiency compared to individual catalysts or a simple catalyst mixture. As a heterogeneous catalyst, TSP-AlCl-PhospBr enables the efficient conversion of CO2 and epoxides, even at low temperatures down to 30 °C, without the use of solvents.
- Phosphonium Salt/Al‐Porphyrin Copolymer as Bifunctional Heterogeneous Catalyst for CO2 Conversion to Cyclic Carbonates,
Laura Valentino, Chloé Célis, Vincenzo Campisciano, Michelangelo Gruttadauria, Carmela Aprile, Francesco Giacalone,
ChemCatChem 2023.
https://doi.org/10.1002/cctc.202301428