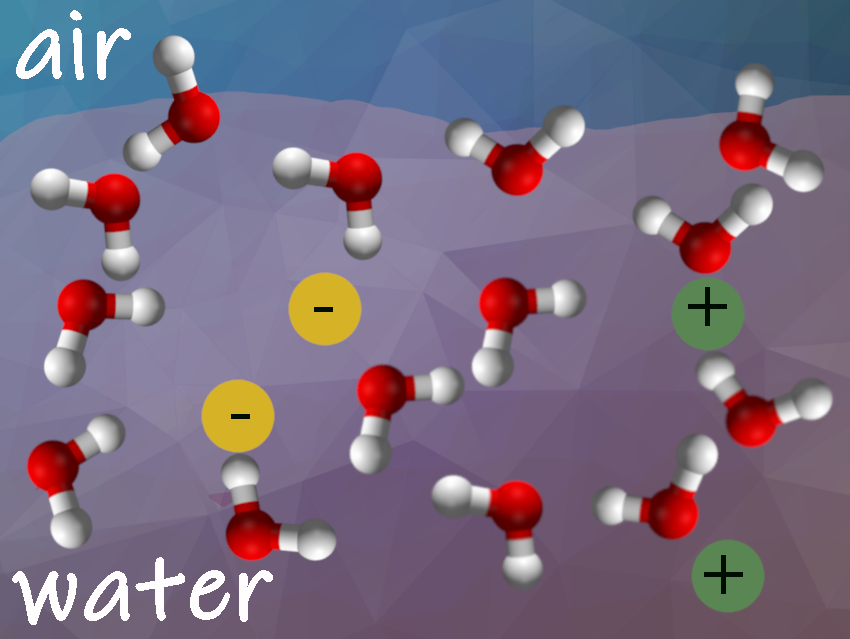
The interface between water and air is only a few molecules thick. Based on previous studies, experts assumed that an electrical double layer of ions forms in typical electrolyte solutions at the interface with air. Polarizable heavy ions, such as Br− and I−, accumulate at the interfacial region, while less polarizable ions, such as F− and Na+, are depleted from the interface. The water molecules then point their positive and negative sides toward these ions. This induces electric fields that determine the interfacial water structure as described in many textbooks.
Yair Litman, Max Planck Institute for Polymer Research, Mainz, Germany, and University of Cambridge, Cambridge, UK, Mischa Bonn, Max Planck Institute for Polymer Research, Mainz, Germany, and colleagues have found that the outermost layer in typical electrolyte solutions consists mostly of pure water. In the layer directly below, the charged particles are highly enriched. The water molecules inside this thin layer, which are next to ions, show a specific orientation along the surface. The researchers studied the structure of the interface of ten electrolyte solutions: HCl, NaOH, CsF, NaF, NaCl, NaBr, NaI, MgCl2, Na2SO4, MgSO4, and NaClO4. Only two solutions made an exception: HCl and NaClO4 formed the electrical double layer. Hydrogen and perchlorate ions had a special tendency to accumulate on the surface.
The team combined high-level heterodyne-detected vibrational sum-frequency generation (HD-VSFG) data with neural network (NN)-aided ab initio molecular dynamics (AIMD) simulations. These insights provide information for solving the puzzle of the air-water interface and understanding chemical reactions in this common environment.
- Surface stratification determines the interfacial water structure of simple electrolyte solutions,
Yair Litman, Kuo-Yang Chiang, Takakazu Seki, Yuki Nagata, Mischa Bonn,
Nature Chemistry 2024.
https://doi.org/10.1038/s41557-023-01416-6