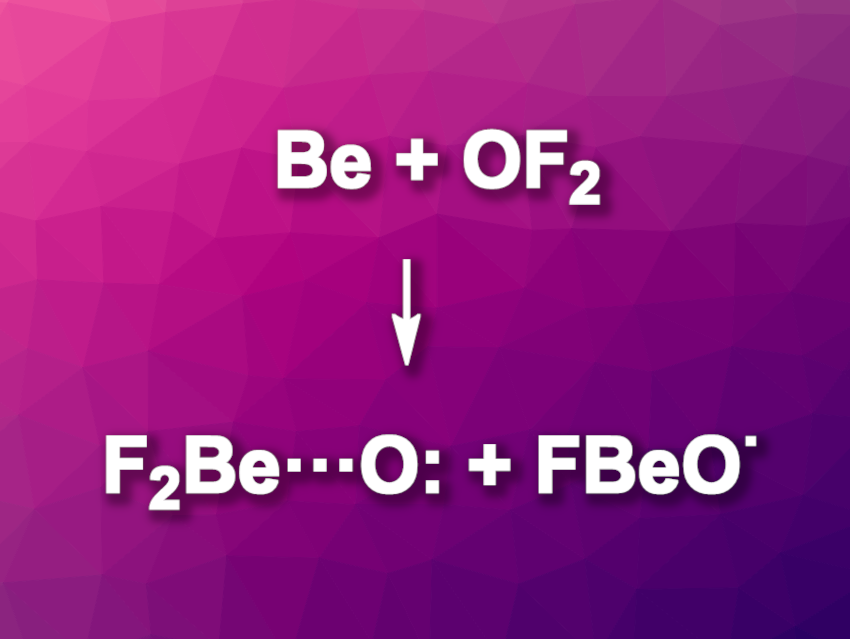Guohai Deng, Sebastian Riedel, Free University Berlin, Germany, Martin Kaupp, Technical University Berlin, Germany, and colleagues have synthesized the beryllium oxyfluoride radicals OBeF and OBeF2 using low-temperature matrix-isolation methods. The team reacted beryllium atoms obtained via laser ablation with OF2 in solid neon and monitored the reactions using infrared (IR) spectroscopy. The results were supported by quantum-chemical calculations and isotope labeling experiments using 18OF2.
The team found that OBeF is linear and has a doublet ground state with an oxyl radical character. OBeF2 has a triplet ground state with an unusual coordinative beryllium–oxygen bond. It represents an unusual case of a triplet oxygen atom stabilized by a relatively weak interaction with the Lewis acid BeF2.
The bonding situation in OBeF2 involves electron donation from the oxygen lone pair to the BeF2 unit as well as back-bonding from the fluorine lone pairs to the empty orbitals at the oxygen. Overall, the work expands beryllium chemistry by two new species.
- Oxygen Atom Stabilization by a Main-Group Lewis Acid: Observation and Characterization of an OBeF2 Complex with a Triplet Ground State,
Guohai Deng, Marc Reimann, Deniz Meyer, Xiya Xia, Martin Kaupp, Sebastian Riedel,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c07079