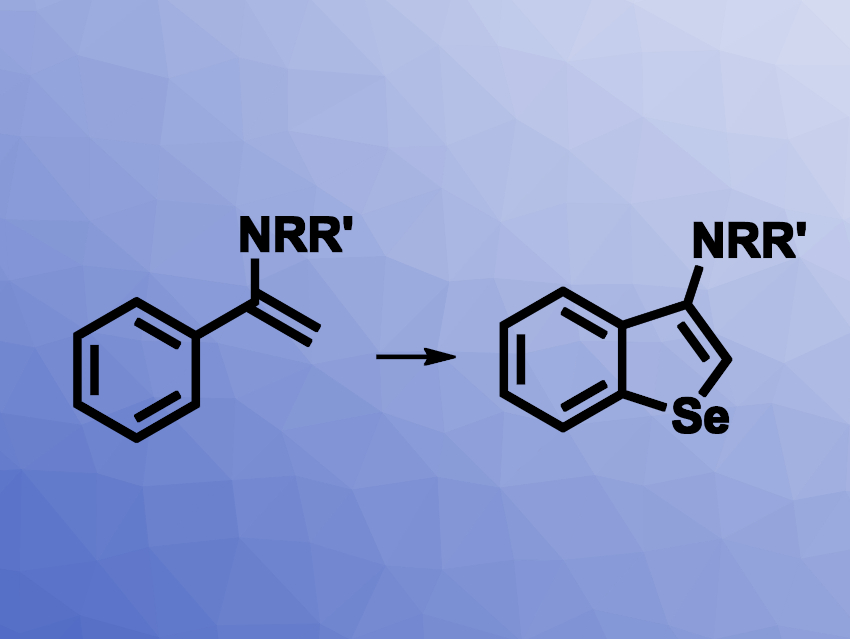
Benzoselenophenes are interesting research targets due to their possible use, e.g., as biologically active compounds or in materials science. Existing methods for their synthesis can have drawbacks such as a need for air-sensitive reagents, starting materials that require multiple-step syntheses, limited substrate scopes, or the disagreeable odors of selenylation reagents. The development of new methods for the preparation of benzoselenophenes would, thus, be useful.
Xiaobao Zeng, Nantong University, China, and colleagues have developed a method for the synthesis of benzoselenophenes from elemental selenium and enamides (pictured) that does not require transition metals, strong oxidants, or difficult-to-prepare starting materials. The team used a variety of functionalized phenyl-substituted enamines as substrates and reacted them with elemental selenium in the presence of trimethylsilyl cyanide (TMSCN), using dimethyl sulfoxide (DMSO) as the solvent. The reactions were performed at 140 °C.
The desired benzoselenophene derivatives were obtained in mostly moderate to good yields. The team also used the method for a gram-scale reaction and obtained the product in 84 % yield.
The researchers propose a reaction mechanism that involves the homolytic cleavage of the Se–Se bonds to form biradical selenium intermediates that can undergo a radical addition with TMSCN. This is followed by the elimination of a trimethylsilyl radical and the formation of an •SeCN radical, which can react with the enamide. The resulting intermediate undergoes a single-electron transfer (SET) process and an elimination of H+ to give a β-selenocyanate. This species is again transformed into a radical under the reaction conditions, and an intramolecular cyclization leads to the desired benzoselenophene.
- Synthesis of Benzoselenophenes via TMSCN-Enabled Radical-Mediated Tandem Reaction Involving Enamides and Elemental Selenium,
Zhenfeng Cheng, Xiaodong Qiu, Biao Xiong, Yanan Zhang, Xiaobao Zeng,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02485



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)