Cyclobutanes are important structural motifs often found in bioactive molecules and natural products. However, their enantioselective preparation has not been widely explored. This can hamper the use of complex cyclobutane derivatives, e.g., in the discovery of synthetic biologically active materials.
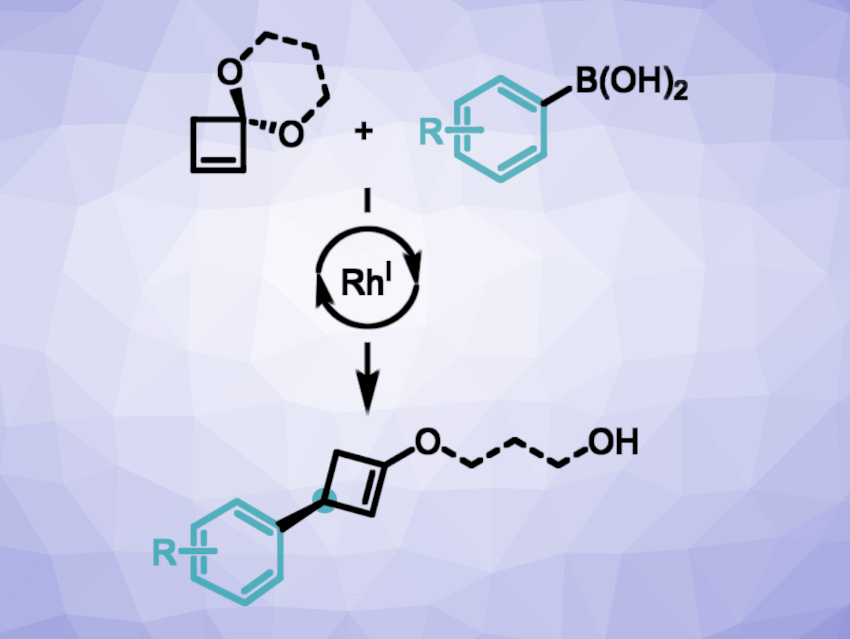
Stephen P. Fletcher, University of Oxford, UK, and colleagues have developed a method for the rhodium-catalyzed enantioselective addition of aryl and vinyl boronic acids to cyclobutenone ketals (general reaction pictured). The team used cyclobutenone ketals as bench-stable cyclobutenone surrogates. They developed a multi-gram synthesis of a 1,3-propanediol-based cyclobutenone ketal starting from commercially available cyclobutanone, which involves an α-bromination, a ketalization, and an elimination.
The cyclobutenone ketal was then reacted with various arylboronic acids and some vinylboronic acids in the presence of [RhCl(coe)2]2 (chlorobis(cyclooctene)rhodium dimer) as the catalyst together with a chiral diene as a ligand, CsOH(aq) as a base, and tetrahydrofuran (THF). Cyclobutenyl enol ethers were obtained in high yields and enantioselectivities. The team found that three other spirocyclic cyclobutenes with different substituents/ring sizes at the ketal were also suitable substrates for the reaction.
The transformation involves an enantioselective carbometalation to give cyclobutyl-rhodium intermediates, followed by β-oxygen elimination to give enantioenriched enol ethers. Overall, this addition can serve as a surrogate for Rh-catalyzed 1,4-additions to cyclobutenone.
- Rhodium‐Catalyzed Asymmetric Arylation of Cyclobutenone Ketals,
David Egea-Arrebola, F. Wieland Goetzke, Stephen P. Fletcher,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202217381



Thanks for the kind highlight! We are really pleased you enjoyed the work.