The structure determination of industrially used 1-chloronaphthalene is of environmental interest due to its toxicity and carcinogenic effects, its formation in the atmosphere, and its potential presence in the interstellar medium. In this study, Susana Blanco, University of Valladolid, Spain, Pablo Pinacho, University of the Basque Country (UPV/EHU), Spain, and colleagues have determined the structure of isolated 1-chloronaphthalene using high-resolution chirped-pulse Fourier transform microwave (CP-FTMW) spectroscopy.
Here they explain why this is important and why it is so complicated.
What have you done?
We investigated the experimental structure of an interesting molecule, 1-chloronaphthalene. It was introduced into a vacuum chamber via a supersonic expansion followed by the application of a short electromagnetic radiation pulse. With that, we made all the molecules of the ensemble rotate coherently.
We recorded the signal from the molecule as its rotational spectrum, which allowed us to derive its rotational constants. These constants are directly related to the three-dimensional shape of the molecule, and are as unique to each conformation as fingerprints.
What is new and cool about this?
The target molecule, 1-chloronaphthalene, is the smallest chlorinated polycyclic aromatic hydrocarbon (PAH). This family of compounds, the PAHs, is of great interest because of their importance in the chemistry of the interstellar medium and their toxicity to humans and the environment. Despite its importance, information on the structure of 1-chloronaphthalene remains scarce, with only a few studies giving an incomplete picture of it.
We present the first accurate experimental structure of 1-chloronaphthalene. By ¨accurate¨, we mean that we have determined the interatomic distances with a precision better than 1 picometer, which is better than 0.000000000001 meters.
How have you done this?
Rotational spectroscopy is a well-established technique spanning over eight decades, and stands as one of the most accurate methods to determine molecular structures. Furthermore, it can also be used to discern the weak intermolecular interactions in complexes comprising two, three, or more molecules.
Characterizing the inherent structure of molecules and how they interact with other compounds, is the first step in understanding their physical, chemical, and biological properties. In addition, the set of measured lines and the determined rotational parameters are necessary to detect the studied molecules in the air and atmosphere and to elaborate and check atmospheric models to analyze their contamination contribution.
What is the main significance of your results?
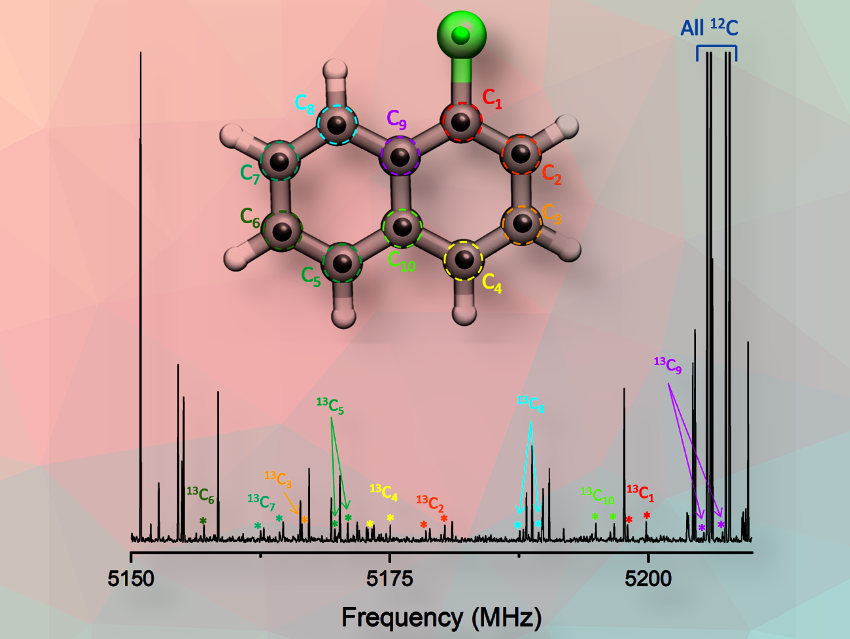
In many rotational spectroscopy studies, not all the monosubstituted heavy atom isotopologues can be observed. This monosubstitution, in which a single atom in the molecule is changed by an isotope while the others remain unchanged is the base to determine the experimental structure.
In our work, we successfully detected signals from the parent (12C, 35Cl), all the 13C isotopologues, as well as from 37Cl. Thus, we could apply different methods to determine the bond lengths and angles within the molecule with great precision. This is the first key finding.
The second discovery is related to the electronic structure of 1-chloronaphthalene. The presence of the chlorine atom alters the electronic structure of the molecule by its inductive (-I) and mesomeric (+M) effects. Thanks to the values of the nuclear quadrupole coupling constants of the chlorine atom, we obtained valuable insights into the electronic structure of the molecule in the surroundings of the chlorine atom.
What is the longer-term vision for your research?
This study laid the foundations for future rotational studies on other polychlorinated naphthalenes (PCNs), and their interactions with other molecules. While several studies have investigated the microsolvation of different PAHs, none has yet explored the microsolvation of polychlorinated naphthalenes. These future studies could shed light on how water and other solvents bind to PCNs, and serve to design potential chemical reactions for their conversion into more benign species or for their removal from the atmosphere.
In addition, as mentioned above, the detection of the studied molecules in air and atmosphere, as well as the analysis of their contamination contribution, requires both the set of measured lines and the determined rotational parameters. These are essential for the development and verification of atmospheric models.
What part of your work was the most challenging?
Our experimental spectra are normally featured by thousands of transitions. Due to the specific conditions of the set-up, it is possible that there are many species present at the same time. Usually, in one run of the experiment, there are many isomers of the target molecule, clusters of different sizes, fragmentation products, isotopologues, …, all of them contributing with their transitions to the spectra.
For this reason, the most challenging part of our work is the analysis of the spectrum to disentangle which signals come from which species. In the specific case of 1-chloronaphthalene, there are more than 550 lines from the main species, another 350 from the 37Cl isotopomer, and around 30 lines for each of the 10 different monosubstituted 13C isotopologues. However, since we see all of them at the same time, the resulting spectrum looks like a forest of lines.
Thank you for these insights.
The article they talked about
- Accurate Experimental Structure of 1-Chloronaphthalene,
Pablo Pinacho, Pablo Gómez, Juan Carlos López, Susana Blancoa,
ChemPhysChem 2023.
https://doi.org/10.1002/cphc.202400072


Pablo Pinacho (pictured on the right) is a postdoctoral researcher at the University of the Basque Country (UPV/EHU), Leioa, Spain, and holds a Maria Zambrano Scholarship funded by the Ministry of Universities.
Pablo Gómez is a M. Sc. from the University of Valladolid, Spain. He participated in this research as part of his Master’s thesis.
Juan Carlos López and Susana Blanco (pictured on the left) are Full Professors from the University of Valladolid, Spain. Both of them are co-leaders of the Rotational Spectroscopy Research Group, RSRG.