Although the concept of mechanochemistry, using mechanical force for chemical reactions, dates back to 314 B.C., the term “mechanochemistry” itself was not officially coined until 1919 by Wilhelm Ostwald, with a concrete definition established six decades later by G. Heinicke, and more recently defined by IUPAC as a reaction induced by direct absorption of mechanical energy. The bead mill technology in a Dyno® mill, also known as an agitator bead mill, commonly used for horizontal wet grinding processes, has never been explored for mechanochemical transformations, either in dry or liquid-assisted grinding conditions.
Evelina Colacino, Université de Montpellier, France, and Ludovic Gremaud, Haute école d’ingénierie et d’architecture de Fribourg (HEIA-FR), Switzerland, and colleagues have used bead milling technology in a Dyno®-mill for the mechanochemical Beckmann rearrangement reaction to synthesize paracetamol in a more sustainable way.
What did you do?
We aimed to use bead-milling technology for the solvent-free synthesis of paracetamol via a mechanochemical Beckmann rearrangement. This chemical pathway is one of the currently used solution-based processes for commercial-scale paracetamol production.
Why are you doing this?
Within the framework of the recently approved Horizon Europe project IMPACTIVE (Innovative Mechanochemical Processes to synthesize green ACTIVE pharmaceutical ingredients), we aim to provide a greener way to access pharmaceuticals and to promote the implementation of mechanochemistry as a greener technology for the large-scale production of active pharmaceutical ingredients (APIs). Indeed, mechanochemistry allows reactions to be carried out without solvents, or at extremely low solvent levels, which account for 75 % of the energy used in the manufacture of APIs.
In a previously reported case study investigation on the continuous flow mechanochemical preparation of the API nitrofurantoin, it was demonstrated that mechanochemical processes can reduce ecotoxicity and CO2 emissions by more than 85 %, while also reducing production costs by 12 %. Therefore, paracetamol was selected as a benchmark and the bead-mill technology was used for the first time to strengthen this approach and provide additional proof-of-concept.
What is new and cool about it?
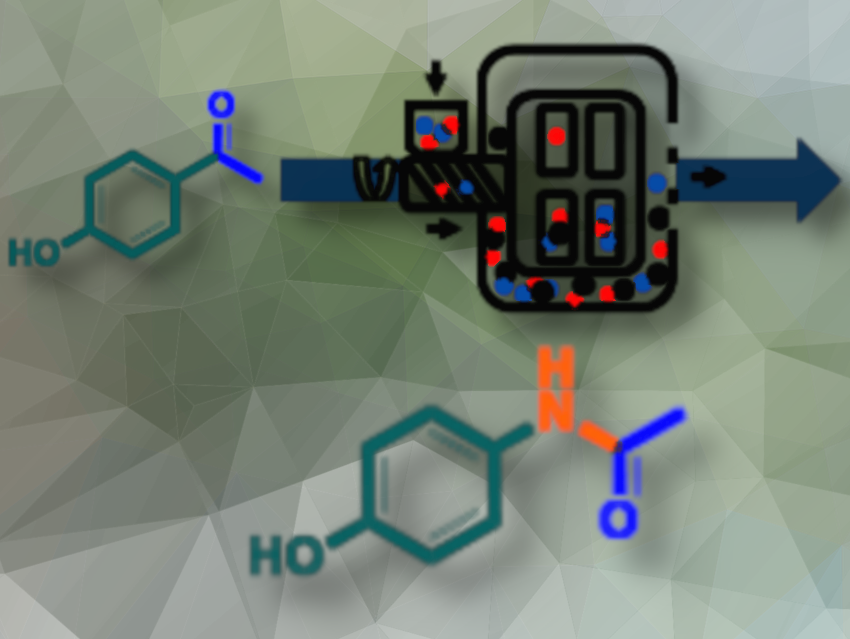
This article highlights the first use of bead-milling technology to perform a reaction in the absence of solvent. For this purpose, an existing device, which normally operates under wet milling conditions, was (re)adapted to operate in batch, semi-continuous, or continuous mode without a solvent (see Fig. 1).
 Figure 1. Schematic representation of the bead-mill technology.
Figure 1. Schematic representation of the bead-mill technology.
What is the main significance of your results?
The first significant result relates to the successful application of bead-milling technology for the mechanochemical synthesis of an API on multi-gram scale. Until now, this technology has only been applied in market sectors other than chemical synthesis. We were able to demonstrate that bead-milling technology applied to the mechanochemical synthesis of paracetamol outperformed solution-based methods, delivering both better yields and green chemistry metrics.
What part of your work was the most challenging?
The most challenging part of our investigation was related to the optimization of the method, since the mechanical stress generated by the device we used was quite different from those generated by the currently available batch or continuous milling machines.
What is the longer-term vision for your research?
Thanks to the encouraging results already obtained within the COST Action CA18112 ‘Mechanochemistry for Sustainable Industry’ (MechSustInd), from which our idea originated, and the results of the IMPACTIVE project, we envisage a long-term implementation of mechanochemical processes for the production of APIs on a commercial scale.
Thank you for the interview.
The paper they talked about
- Sustainable Beckmann Rearrangement using Bead-Milling Technology: The Route to Paracetamol,
Romain Geib, Evelina Colacino, Ludovic Gremaud,
ChemSusChem 2024.
https://doi.org/10.1002/cssc.202301921
 |
 |
|
Dr. Ludovic Gremaud |
Dr. Evelina Colacino |
Also of Interest
The video explains the potential of mechanochemistry for greener pharmaceuticals. IMPACTIVE is a project funded by the European Union.