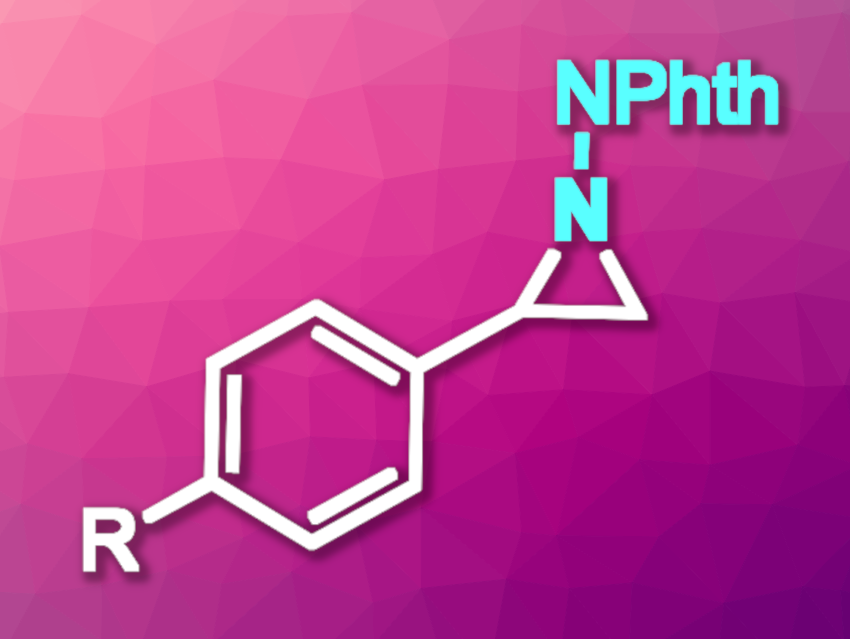
Aziridines, i.e., three-membered heterocycles with one nitrogen atom, are useful intermediates in organic synthesis and can also be found in a number of biologically active compounds. The development of new methods for the synthesis of these strained heterocycles is an interesting research topic. One type of approach is the [2+1] cycloaddition of reactive nitrene intermediates with alkenes. Metal-free reactions, in particular, could be interesting, cost-effective options in this context.
Marvin Parasram, New York University, NY, USA, and colleagues have developed a method for the photoinduced aziridination of alkenes that uses phthalimide-protected azoxy-triazenes as nitrogen atom sources. The team reacted 1-phenyl-2-phthalimidodiazene-1-oxide with a variety of styrene derivatives (example product pictured) or different unactivated alkenes under light irradiation (390 nm) in 1,4-dioxane at room temperature.
Under these conditions, the desired aziridine derivatives were obtained in mostly moderate to high yields. The reaction proceeds under mild conditions, is chemoselective, and tolerates a wide range of functional groups. The team successfully scaled up the reaction to the gram scale. They also adapted it to flow conditions, which improved the transformation’s productivity for substrates with comparatively low yields in the batch process. Mechanistic studies supported the hypothesis that the reaction involves the fragmentation of the azoxy-triazene to generate a free singlet nitrene.
- Aziridination via Nitrogen-Atom Transfer to Olefins from Photoexcited Azoxy-Triazenes,
Joshua K. Mitchell, Waseem A. Hussain, Ajay H. Bansode, Ryan M. O’Connor, Marvin Parasram,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c14713