π-Conjugated macrocycles can show different types of aromaticity. For example, annulenes, which are monocyclic hydrocarbons with alternating single and double bonds such as porphyrin can display global aromaticity. In contrast, π-conjugated macrocycles based on benzene rings, such as cyclo-para-phenylenes (CPPs), typically show only local aromaticity. The introduction of quinoidal units into such benzene-unit-based macrocycles could lead to effective electron delocalization and global (anti)aromaticity.
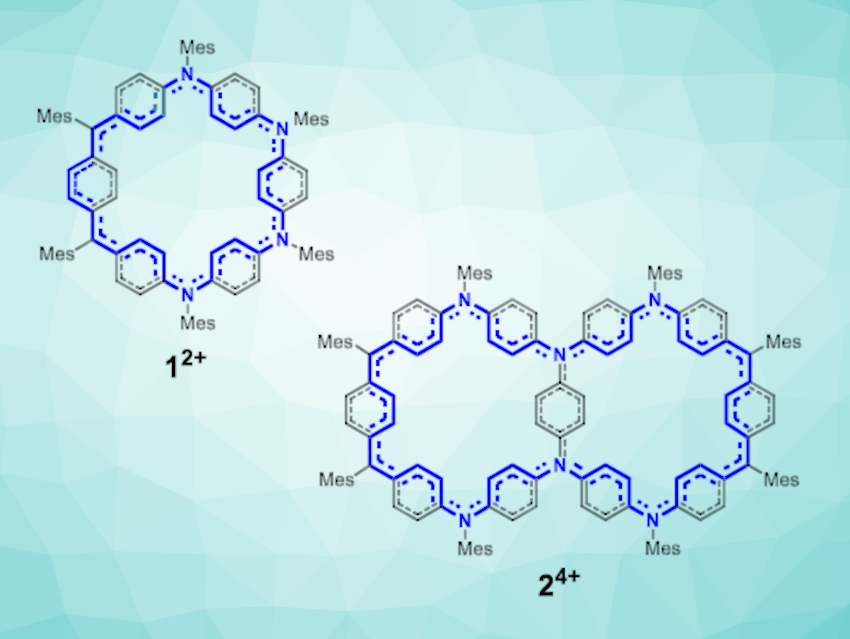
Chunyan Chi, National University of Singapore, and colleagues have synthesized two fully π-conjugated macrocycles that contain para-quinodimethane and triphenylamine units, a tetraazasuperbenzene and a hexaazasupernaphthalene. The team used intermolecular Friedel-Crafts alkylation reactions to build the overall macrocyclic structures, followed by oxidative dehydrogenation to generate the desired π-conjugated products.
The researchers found that both products have nearly planar conformations with localized aromaticity. However, when they are oxidized, the resulting cations show global (anti)aromaticity. The tetraazasuperbenzene-based dication and tetracation display global anti-aromaticity (with 32 π electrons) and aromaticity (30 π electrons), respectively (dication pictured).
For the hexaazasupernaphthalene, the corresponding dication, tetracation, and hexacation show global aromaticity (with 54 π electrons), antiaromaticity (52 π electrons), and aromaticity (50 π electrons), respectively (tetracation pictured). According to the team, the work provides valuable insights for designing and synthesizing more complex macrocycle-based structures in the future.
- Facile Synthesis and Global Aromaticity of Aza‐Superbenzene and Aza‐Supernaphthalene at Different Oxidation States,
Yunhan Ma, Yi Han, Xudong Hou, Shaofei Wu, Chunyan Chi,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202407990

![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)


good job