The difunctionalization of alkenes using asymmetric, three-component reactions provides a path to rapidly construct complex molecules in organic synthesis. This type of reaction can use a combination of transition-metal catalysis and radical chemistry, for example, using acyl radicals to give carbonyl-functionalized products. However, finding methods for such asymmetric acylative difunctionalizations that directly give β-branched chiral ketones from alkenes has remained challenging so far.
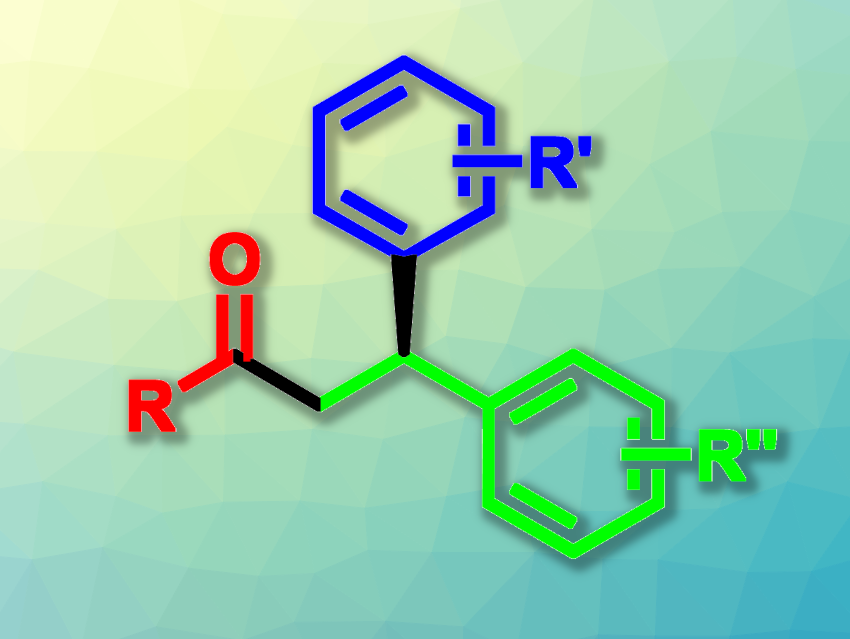
Dong Xing, East China Normal University, Shanghai, China, and colleagues have developed a copper-catalyzed asymmetric acylarylation of alkenes using aldehydes and aryl boronic acids (general product structure pictured). The team used Cu(CH3CN)4BF4 as the copper catalyst together with a chiral binaphthyl-tethered bisoxazoline ligand, tert-butyl hydroperoxide (TBHP) as a radical initiator, KHCO3 as a base, Zn as an additive, and acetonitrile as the solvent. The reactions were performed at 40 °C. A variety of vinylarenes (unit pictured in green) were reacted with different aldehydes (unit pictured in red) and a range of aryl boronic acids (unit pictured in blue).
Under these conditions, the desired difunctionalized products were obtained in moderate to high yields and with generally good enantioselectivities. These products can be used in further transformations, e.g., to prepare drug-like molecules. According to the researchers, the reaction proceeds via the generation of an acyl radical from the aldehyde, followed by radical addition to the vinylarene and copper-catalyzed arylation. Overall, the work provides a method for the synthesis of enantioenriched chiral β, β-diaryl ketones.
- Cu-Catalyzed Asymmetric Three-Component Radical Acylarylation of Vinylarenes with Aldehydes and Aryl Boronic Acids,
Zhiheng Li, Shang Wang, Si-Cong Chen, Xiangwen Zhu, Zhengzhen Lian, Dong Xing,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c08957