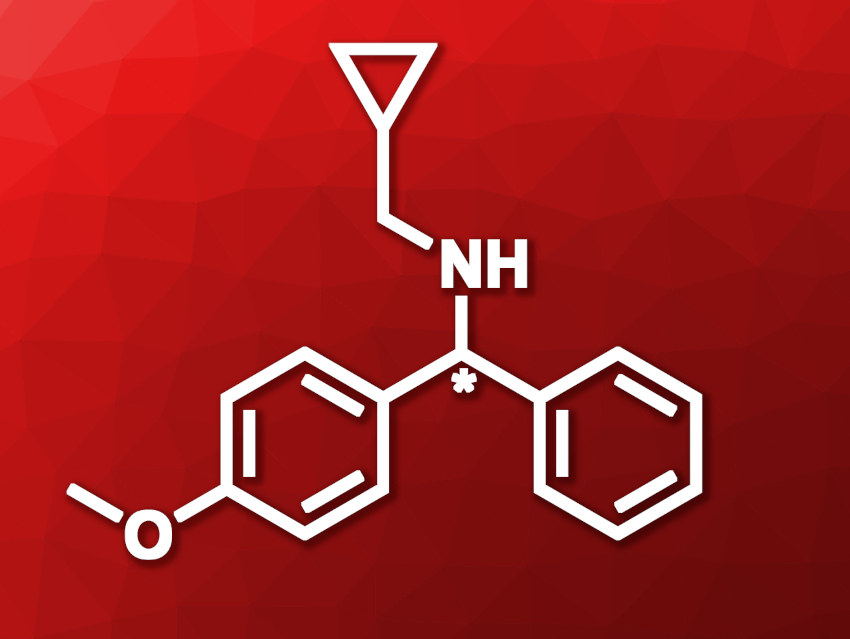
Chiral N-substituted 1-aryl-1-phenylmethylamines (example pictured) can be useful structures in pharmaceutical chemistry. They can be obtained, e.g., using metal catalysis, but this approach requires chiral ligands and can involve expensive metals and harsh reaction conditions, which has hampered its use. Enzymatic reactions could be an alternative. Imine reductases (IREDs), for example, could theoretically be used to convert N-cyclopropylmethyl-1-aryl-1-phenylmethylimines to the desired amines. However, such bulky substrates can be difficult to convert, and suitable enzyme variants need to be found.
Qi Wu, Zhejiang University, Hangzhou, China, and colleagues have developed an engineered IRED for the enantioselective reduction of different N-cyclopropylmethyl-1-aryl-1-phenylmethylimines. The team started from an IRED from Nocardia cyriacigeorgica and changed amino acid residues located in the enzyme’s catalytic pocket using a strategy called “focused rational iterative site-specific mutagenesis” (FRISM) to generate a small, targeted series of enzyme candidates. They screened the obtained enzymes and found two variants that provide improved yields and enantioselectivities compared with the wild-type enzyme.
The researchers used the promising enzyme variants for the conversion of various N-cyclopropylmethyl-1-aryl-1-phenylmethanimines. The enzymes showed a broad substrate scope. The team obtained the desired amine products with bulky substituents in moderate to high yields and ee values of up to 99 %.
- Asymmetric Synthesis of Bulky N-Cyclopropylmethyl-1-aryl-1-phenylmethylamines Catalyzed by Engineered Imine Reductases,
Haonan Zhou, Peihsuan Chuang, Leyan Xu, Qi Wu,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02542