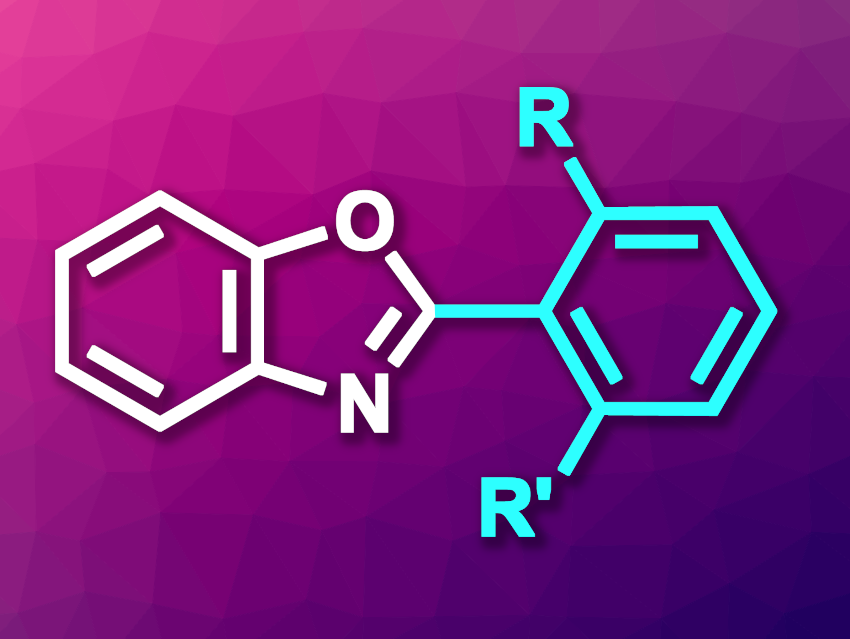
Benzoxazoles are fused heterocycles that have applications, e.g., in materials science and and drug discovery. Benzoxazoles with a 2,6-disubstituted arene unit at the C2 position that features bulky substituents, which limit rotation, can be particularly interesting, e.g., for use in light-emitting and luminescent materials. Such substituted benzoxazole units can also be found in biologically active molecules.
Chi Wai Cheung, Pui Ying Choy, Fuk Yee Kwong, The Chinese University of Hong Kong, Shatin, China, and colleagues have developed a method for the palladium-catalyzed C2–H arylation of benzoxazoles using sterically hindered aryl chlorides (general product structure pictured). The team used a new indolylphosphine ligand for the transformation. The reacted different benzoxazole derivatives with 2-chloro-m-xylenes and other 1,3-substituted 2-chloroarenes in the presence of Pd(OAc)2 and the phosphine ligand as the catalytic system and LiOtBu as a base. The reactions were performed in tetrahydrofuran (THF) as a solvent at 110 °C.
Under these conditions, the desired products were obtained in good to high yields. Overall, the work allows the efficient synthesis of sterically congested 2,6-disubstituted 2-arylbenzoxazoles. According to the researchers, they will work on other applications of their newly developed ligand in direct C–H arylation processes with high steric hindrances.
- A General Pd-Catalyzed C2–H Arylation of Benzoxazoles with Highly Sterically Congested Aryl Chlorides Enabled by a Fittingly Tuned Ligand,
Weijian Song, Man Ho Tse, Yu Kiu Lau, Chi Wai Cheung, Pui Ying Choy, Fuk Yee Kwong,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c04117



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)