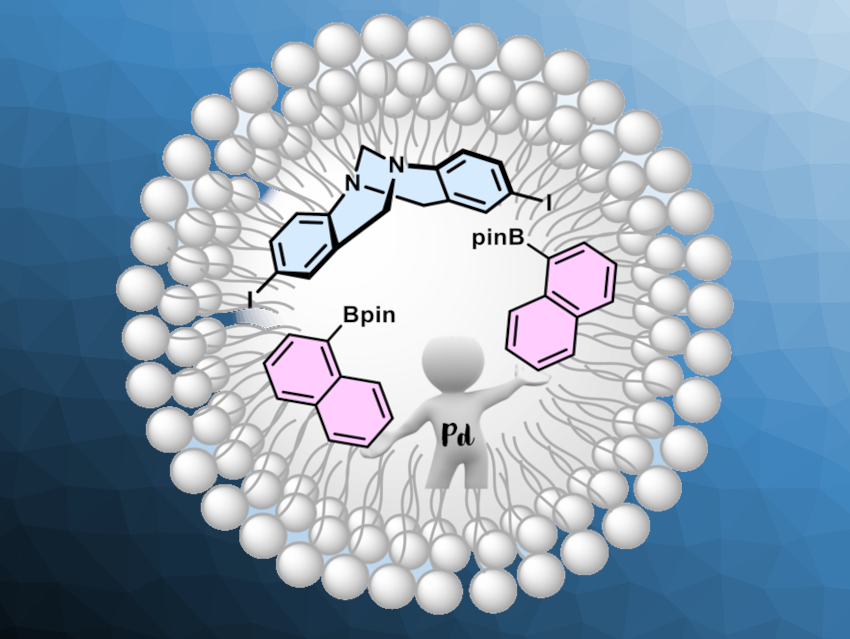
Traditionally, organic reactions are carried out in organic solvents due to the solubility of the reagents. In some cases, it could be useful to imitate biosynthesis processes that take place in living organisms, where organic reactions are carried out in water. Surfactants can be helpful in such processes and form micellar aggregates that act as a nanoreactor. Tröger’s base (iodo-derivative pictured in blue above) is a molecule with an uncommon V-shaped structure that features two aromatic planes. It can be useful, e.g., in molecular sensors. However, the synthesis of a broad range of arylated Tröger’s base derivatives is challenging.
Angélica V. Moro, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, and colleagues have developed a method for the arylation of Tröger´s base via a Suzuki cross-coupling reaction using aqueous micellar catalysis. The team reacted iodo-Troger’s base with different arylboronates in the presence of SPGS-550-M as the surfactant in water, using low loadings of palladium acetate as the catalyst. The micellar catalytic system can be applied to a broad range of electron-poor pinacolboronates and tolerates various functional groups, such as CF3, cyano, ester, and aldehyde groups. Electron-richer arylboronates were found to be less well-suited as substrates, with reduced yields or without the formation of the desired products.
The developed approach is in accordance with “green chemistry” principles: It uses a low catalyst loading and an environmentally friendly reaction medium, which decreases the need for the use of organic solvents. The products obtained by this protocol have interesting photophysical properties and potential applications, e.g., as fluorescent sensors.
- Suzuki Coupling in Tröger’s Bases: Overcoming Challenging Substrates through Aqueous Micellar Catalysis,
Eduam O. Boeira, Caroline B. Plá, Fabiano S. Rodembusch, Angélica V. Moro,
ChemCatChem 2023.
https://doi.org/10.1002/cctc.202201355