Infections caused by Pseudomonas aeruginosa (PA), a Gram-negative pathogen, are a leading cause of death in cystic fibrosis patients. PA strains that are resistant to first-line antibiotic treatments are on the rise. One of the challenges in developing antibiotics that target Gram-negative pathogens like PA is penetrating the negatively charged outer membrane, which limits antibiotic accumulation in the cell. Nitrogen-based functional groups such as amines, guanidines, and amidines, which can be positively charged at physiological pH, help to increase antibiotic uptake in Gram-negative bacteria.
Amanda L. Wolfe, University of North Carolina, Asheville, USA, and colleagues have synthesized a series of dimeric small molecules with benzamidine, benzguanidine, dimethyl amine, and pyridine units. The team used an efficient triphosgene-mediated dimerization reaction to obtain the desired compounds. They examined the compounds’ activity against multidrug-resistant PA, which were isolated from cystic fibrosis patients.
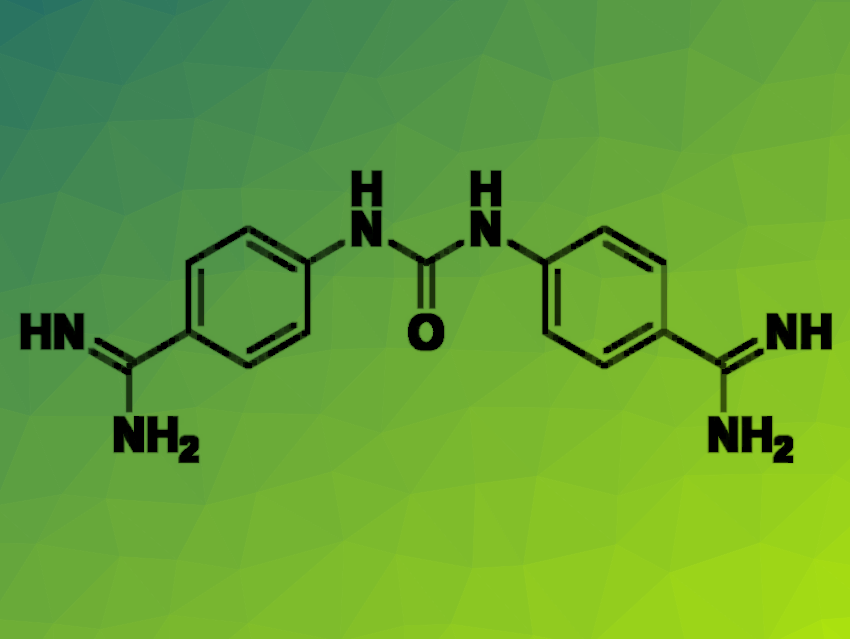
The researchers found that 4,4′-(carbonylbis(azanediyl))dibenzimidamide dihydrochloride (neutral form pictured) and 1,3-bis(4-guanidinophenyl)urea dihydrochloride were capable of inhibiting several of the tested strains. While both molecules have been previously found to have antiparasitic activity, this is the first report of their activity against PA, demonstrating the value in wide biological evaluation of known compounds.
- Bisbenzamidine and Bisbenzguanidine Ureas Act as Antibacterial Agents Against Pseudomonas aeruginosa,
Casey N. Kellogg, Bryce A. Pugh, Isaak M. Starr, Dhruvi J. Parmar, A’Zane D. Troxler, Amanda Wolfe,
ChemMedChem 2023.
https://doi.org/10.1002/cmdc.202300496