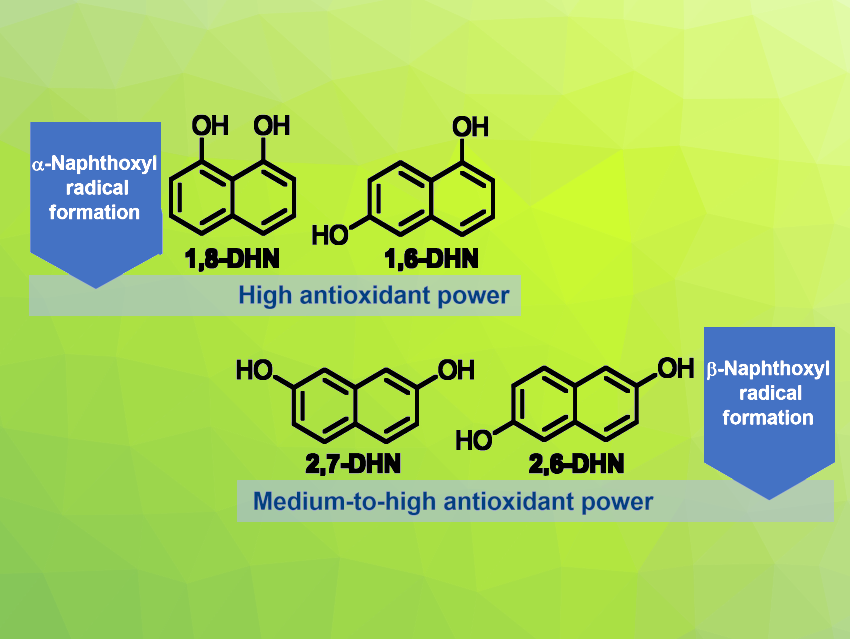
Efficient, sustainable, and biocompatible antioxidant systems can have many applications, ranging from the biomedical to the food and materials sectors. Natural structural motifs with superior hydrogen atom donor and/or electron transfer properties could be useful in this context. 1,8-dihydroxynaphthalene (1,8-DHN), for example, is a naturally occurring polyketide derivative found in various fungi. This compound has outstanding antioxidant properties, which might be due to the stabilization of aryloxyl radicals resulting from hydrogen atom transfer (HAT) via intramolecular hydrogen bonding and delocalization.
Paola Manini, University of Naples Federico II, Napoli, Italy, and colleagues have investigated the potential of hydroxylated naphthalenes as an antioxidant platform and looked for relationships between structure and antioxidant properties. The team used a combined experimental and theoretical approach and compared four isomers (pictured above): 1,8-DHN, 1,6-dihydroxynaphthalene (1,6-DHN), 2,6-dihydroxynaphthalene (2,6-DHN), and 2,7-dihydroxynaphthalene (2,7-DHN). They also compared these compounds to 1-naphthol (1-HN) and 2-naphthol (2-HN) as references. Antioxidant assays, laser flash photolysis studies, and the isolation of oligomeric intermediates from oxidative polymerization processes were used to compare these species, in addition to computational studies.
The team found that a higher antioxidant power and faster HAT processes are associated with an α-substitution pattern, as in 1,8-DHN and 1,6-DHN, compared with DHNs with a β-substitution pattern like 2,6-DHN and 2,7-DHN. According to the researchers, the main factors governing the antioxidant activity of DHNs are the generation and fate of the intermediate naphthoxyl radicals. The work could be useful for the design of next-generation antioxidants.
- Antioxidant Activities of Hydroxylated Naphthalenes: The Role of Aryloxyl Radicals,
Valeria Lino, Paola Manini, Marco Galeotti, Michela Salamone, Massimo Bietti, Orlando Crescenzi, Alessandra Napolitano, Marco d’Ischia,
ChemPlusChem 2023.
https://doi.org/10.1002/cplu.202200449