Our immune system is always on alert, detecting and eliminating pathogens and cancer cells. Cellular control mechanisms cause diseased cells to present antigens on their surface, like signs for the immune system. For analysis of the necessary complex antigen processing and transport processes in real time, Robert Tampé, Ralph Wieneke, Goethe University Frankfurt, Frankfurt am Main, Germany, and colleagues have developed a “cage” that is opened with light to release trapped antigens at a specific place and time.
Antigen Processing and Presentation
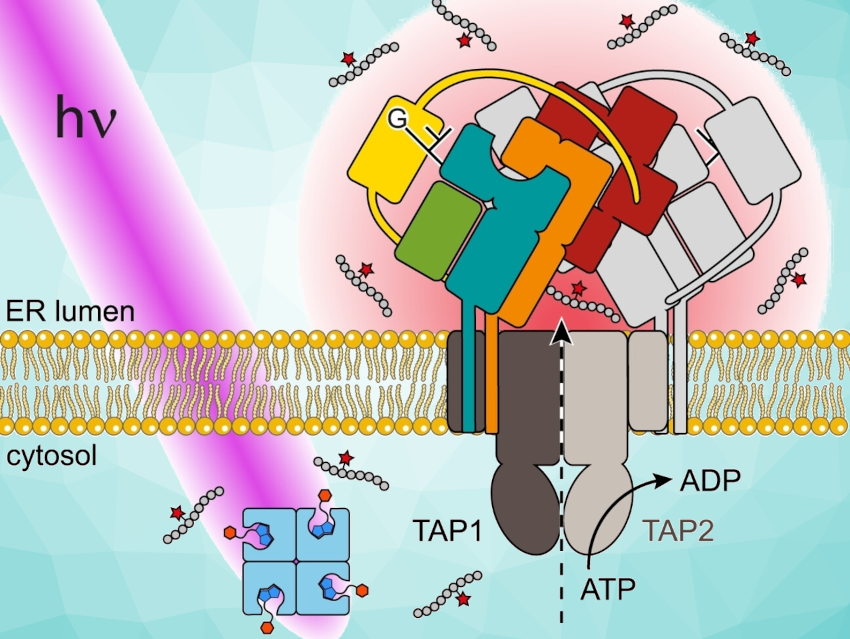
In our cells, both our own and foreign proteins are constantly broken into tiny pieces and transported into the endoplasmic reticulum (ER), a branched system of channels enclosed by a membrane, by the transporter associated with antigen processing (TAP). There, the supramolecular peptide loading complex (PLC) controls the loading of MHC I (major histocompatibility complex class I) with antigenic peptides.
Certain peptides are preferentially loaded onto MHC I, further processed for immune surveillance (antigen processing) and presented on the cell surface. Peptides that come from normal endogenous proteins remain immunologically inconspicuous (unless there are misdirected autoimmune reactions).
On-Demand, Photostimulated Antigen Release
Despite many new insights, the mechanistic principles of antigen translocation, dynamic PLC assembly, and the interaction between different PLC subunits in the “quality control” of peptide-MHC complexes remain generally unknown so far. To further analyze antigen processing, the team developed a photostimulated antigen release system that can be used to precisely study antigen flux.
The antigens are released in the form of an “antigen burst” on command from a “caged”, inactive state by irradiation with light. The advantage of light stimulation is that it can be precisely dosed at limited times and locations and is noninvasive, which allows for experiments in living cells.
Steric Hindrance Used to Block Recognition
The team used a peptide derived from an HIV antigen as their model. They used a linker to bind the epitope (section of an antigen) to biotin and then the biotin to a voluminous protein called streptavidin. In this state, the epitope is shielded to the extent that it can no longer be recognized by the antigen processing transporter (TAP).
The linker contains a group that can be split apart by light. When irradiated with UV light, the peptide epitope is immediately released from its cage. It is then recognized by TAP, transported across the ER membrane, and loaded onto MHC I by way of the PLC.
Versatile Framework for Controlling the Supply of Antigens
This method is versatile, as demonstrated by a variety of scenarios, such as tracing antigen transport by the TAP of native human PLC in the ER membrane of a human lymphoma cell line. According to the researchers, “it is our aim to use the light-activated system to follow the antigen processing pathway through different cellular compartments and gain an understanding of the kinetics of various immunological processes in vivo.”
- Antigen Delivery Controlled by an On‐Demand Photorelease,
Max Löffler, Stefan Frühschulz, Zoe Rockel, Matija Pečak, Robert Tampé, Ralph Wieneke,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202405035