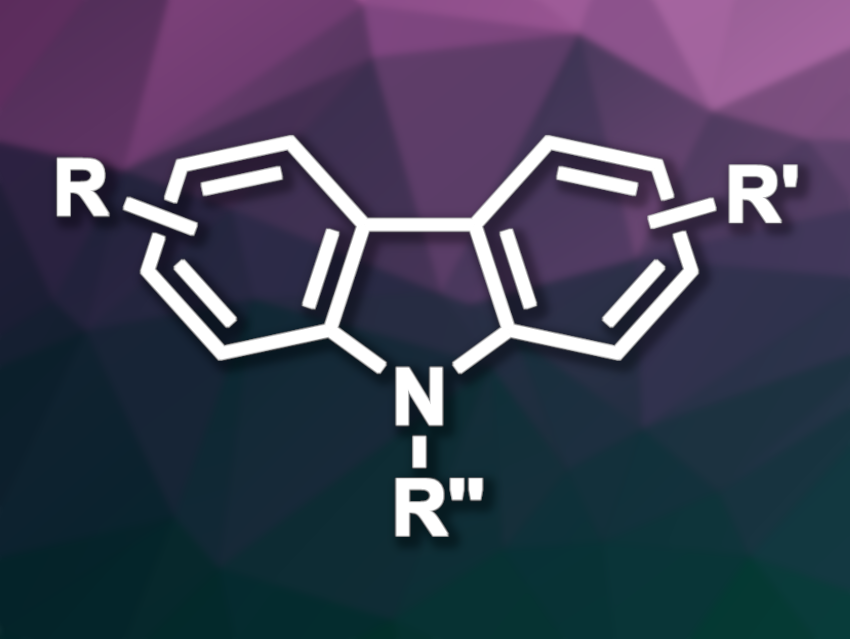
Carbazoles are nitrogen-containing heterocycles composed of two benzene rings fused to a central pyrrole ring. One approach to their synthesis is using 1,2,3,4-tetrahydrocarbazoles, which can be synthesized from aryl hydrazines and ketones, and subjecting them to an oxidative aromatization to obtain the desired carbazoles. However, the oxidation step often requires high temperatures and stoichiometric amounts of oxidants.
Eisuke Sato, Seiji Suga, Okayama University, Japan, and colleagues have developed an electrochemical method for the bromide-mediated anodic aromatization of protected 1,2,3,4-tetrahydrocarbazole to give carbazoles. The team used, e.g., N-acetyl-1,2,3,4-tetrahydrocarbazole derivatives as substrates, low-cost LiBr as the halogen mediator, AcOH as a sacrificial proton source, MeCN as the solvent, and an undivided cell with Pt electrodes. The reactions were performed at 70 °C.
The desired carbazoles were obtained in moderate to good yields. The team proposes a reaction mechanism that involves the generation of a bromonium ion equivalent, probably tribromide, which reacts with the substrate to generate an iminium intermediate. Elimination of HBr and a proton then gives an oxidized intermediate, and repeating this process gives the desired aromatic product.
- Anodic Dehydrogenative Aromatization of Tetrahydrocarbazoles Leading to Carbazoles,
Eisuke Sato, Ayaka Yukiue, Koichi Mitsudo, Seiji Suga,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c01914


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
