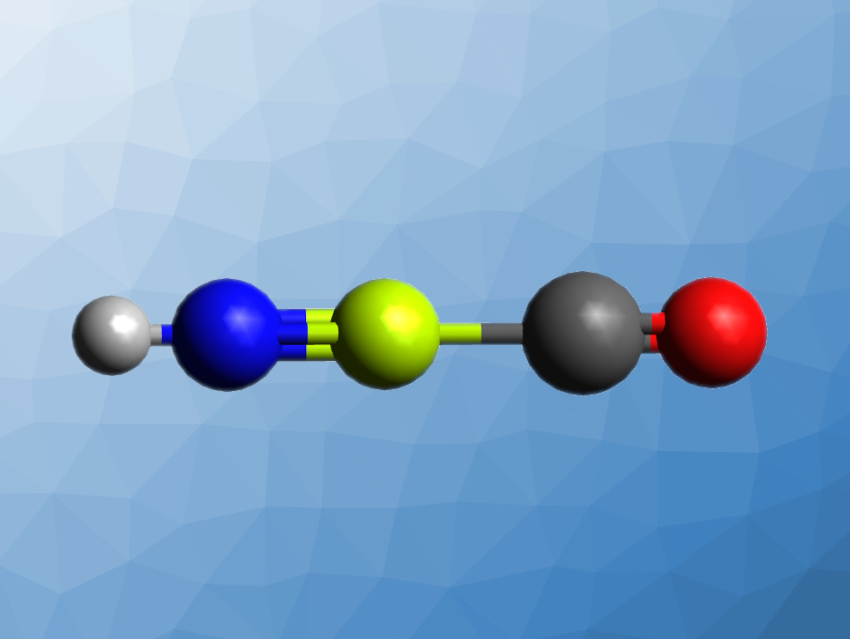
Multiple bonds are common for elements such as carbon and other p-, d- and f-block elements. Elements in the s-block of the periodic table, such as the alkali metals and alkaline earth metals, mostly form ionic compounds. It is very difficult for them to form multiple bonds. Nevertheless, the possibility of double and triple bonding has been predicted theoretically, and there have been a few examples of beryllium compounds with at least some multiple-bond character.
Mingfei Zhou, Shanghai Key Laboratory of Molecular Catalysts and Innovative Materials, Fudan University, China, Gernot Frenking, University of Marburg, Germany, and Nanjing Tech University, China, and colleagues have found an unprecedented N≡Be triple bond in HNBeCO (pictured). The team prepared HNBeCO via a reaction of laser-evaporated beryllium atoms with isocyanic acid (HNCO) in a solid neon matrix. HNCO was synthesized by heating a mixture of stearic acid and sodium cyanate to 90 °C.
The product was characterized using infrared (IR) spectroscopy, and its structure and properties were investigated using both ab initio quantum chemistry methods and density functional theory (DFT) calculations. The team found that the product has a linear structure with an N≡Be triple bond that involves two stronger, electron-sharing π bonds and a weak, dative σ bond.
- Triple Bonding Between Beryllium and Nitrogen in HNBeCO,
Lina Wang, Sudip Pan, Guanjun Wang, Xiaoqing Zeng, Mingfei Zhou, Gernot Frenking,
Chem. Commun. 2022.
https://doi.org/10.1039/d2cc02969c