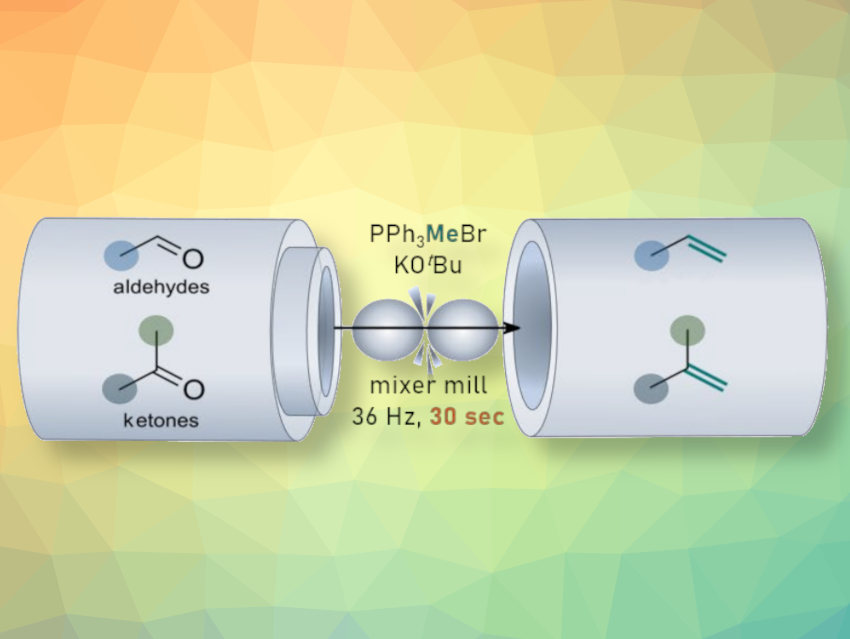
The Wittig olefination converts carbonyl compounds into the analogous alkenes. It is an important, widely used transformation in organic synthesis. More sustainable variants of this type of reaction could, thus, be useful for the transformation to green chemistry. Mechanically induced, solvent-free reactions are promising in this context.
Michael Schnürch, Vienna University of Technology (TU Wien), Austria, and colleagues have developed a protocol for an ultrafast, solvent-free, mechanically induced Wittig reaction under ambient conditions (general reaction pictured). The team reacted a range of aldehydes and ketones with different phosphonium salts in the presence of KOtBu as a base under high-energy ball milling conditions. The reactions were performed in polytetrafluoroethylene (PTFE) milling vessels containing one hardened steel ball with a size of 12 mm, at a defined frequency of 36 Hz. The milling time can be as short as 30 s.
The method eliminates the need for strict air and moisture exclusion for a range of substrates and provides a simpler, more sustainable, and efficient alternative to the corresponding solution-phase reactions. The team found that the protocol can be used for the conversion of a diverse range of carbonyl compounds into the corresponding olefins with high yields.
- High‐Energy Ball Milling Enables an Ultra‐fast Wittig Olefination Under Ambient and Solvent‐free Conditions,
Johanna Templ, Michael Schnürch,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202411536