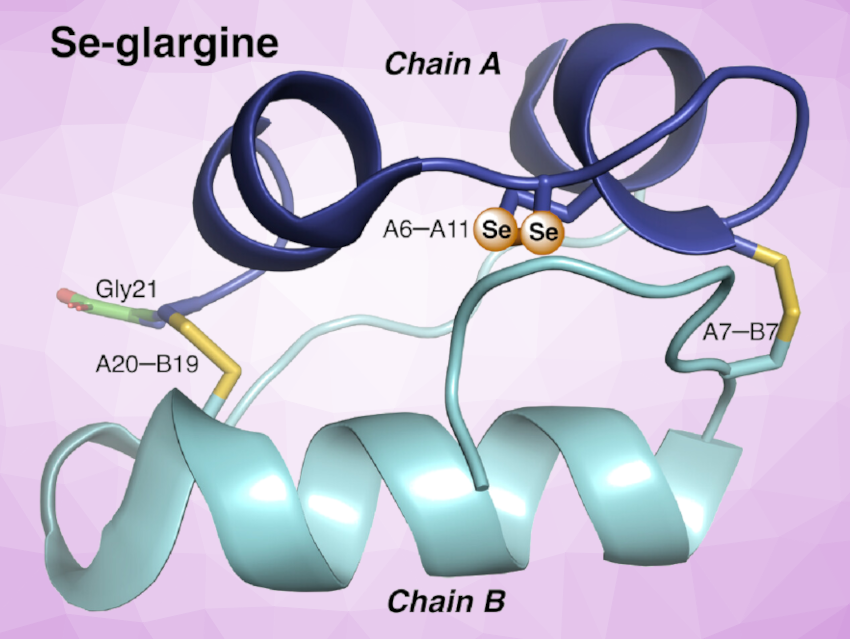
The chemical synthesis of insulin can be challenging due to its two-chain structure (chains A and B, see picture) with a specific pattern of disulfide pairing. There are two interchain (A7–B7 and A20–B19) and one intrachain (A6–A11) disulfide bridges. Traditional synthetic approaches can be hampered by slow and inefficient disulfide pairing. In addition to easier preparation methods, synthetic insulin formulations with higher stabilities are interesting research targets. The insulin glargine, for example, is one of the most prescribed long-lasting analogues in the management of both type 1 and type 2 diabetes.
Michael A. Weiss, Indiana University School of Medicine, Indianapolis, USA, Norman Metanis, The Hebrew University of Jerusalem, Israel, and colleagues have synthesized the seleno-analogue of glargine (Se-glargine, pictured) wherein CysA6 and CysA11 (paired to form the internal A6–A11 disulfide bridge) were substituted by selenocysteine. Interestingly, this A6–A11 diselenide substitution considerably enhanced the rate and yield of chain combination. Se-glargine was obtained in a yield that permitted isolation, characterization, and analytical studies within a few hours, whereas control reactions using the unmodified glargine A chain gave negligible yields.
The Se-glargine analogue retained a native-like structure and activity. In addition, the diselenide bridge enhanced the thermodynamic stability of the anlogue. This enhanced stability could have advantages such as extending Se-glargine’s shelf life and delaying its rate of physical degradation above room temperature. According to the researchers, studies on this are in progress.
- Se‐Glargine: Chemical Synthesis of a Basal Insulin Analogue Stabilized by an Internal Diselenide Bridge,
Orit Weil‐Ktorza, Balamurugan Dhayalan, Yen‐Shan Chen, Michael A. Weiss, Norman Metanis,
ChemBioChem 2024.
https://doi.org/10.1002/cbic.202300818