The classic Finkelstein reaction involves a halide exchange and is used for the preparation of alkyl iodides from alkyl bromides or chlorides. Analogously, the copper-catalyzed aromatic Finkelstein reaction exchanges a Br substituent at an aromatic ring with an I substituent. An enantioselective variant of the aromatic Finkelstein reaction had not been reported so far.
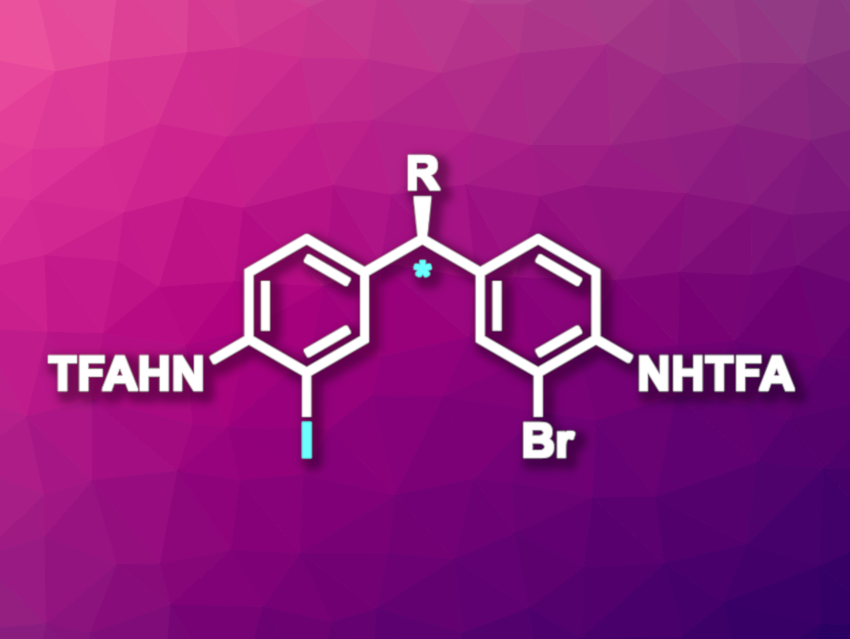
Matthew S. Sigman, University of Utah, Salt Lake City, USA, Scott J. Miller, Yale University, New Haven, CT, USA, and colleagues have developed an enantioselective aromatic Finkelstein reaction that can be used for the remote desymmetrization of diarylmethanes (general product structure pictured, TFA = trifluoroacetyl). The reaction is copper-catalyzed and uses a guanidinylated peptide ligand. In the transformation, one of two Br substituents is selectively replaced with an I substituent, giving chiral products.
The team used Cu(MeCN)4BF4 as the catalyst and a tetrameric β-turn peptide as the ligand together with K3PO4 and NaI for stereoselective bromide-to-iodide substitutions in a range of achiral, dibromosubstituted diarylmethanes. The resulting iodinated products were not isolated, but can be further transformed using a variety of transformations that selectively occur at the C–I bond over the C–Br bond and give useful products. The team used, e.g, a Heck reaction with ethyl acrylate, a Larock-type indole formation, or a Suzuki reaction with 3-methoxyphenylboronic acid.
- An Asymmetric Aromatic Finkelstein Reaction: A Platform for Remote Diarylmethane Desymmetrization,
Tobias Morack, Tyler E. Myers, Lucas J. Karas, Melissa A. Hardy, Brandon Q. Mercado, Matthew S. Sigman, Scott J. Miller,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c08727



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)