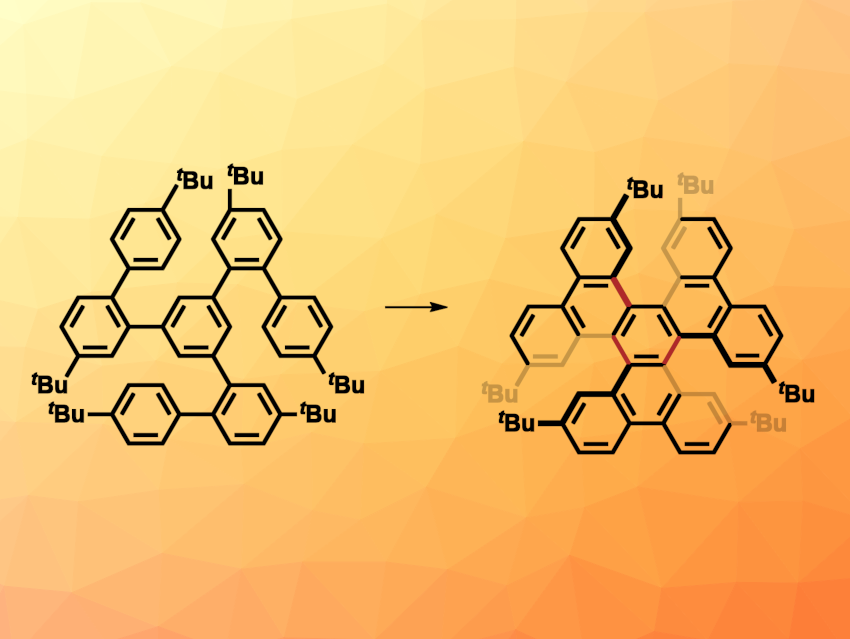
Polycyclic aromatic hydrocarbons (PAHs) with distorted structures can have interesting and useful optoelectronic properties. Their non-planar structures can reduce the tendency to aggregate and allow solution processing. The Scholl reaction is often used to transform flexible precursors into rigid PAHs by forming multiple new carbon–carbon bonds between aromatic rings. The conventional Scholl reaction often requires superstoichiometric amounts of oxidants. Electrochemical reactions could serve as greener alternatives.
Yuanming Li, Ke-Yin Ye, Fuzhou University, China, and colleagues have developed an electrochemical continuous-flow Scholl reaction for the preparation of PAHs (example reaction pictured). The team performed electrolysis in a continuous-flow cell with a carbon graphite anode, a nickel cathode, a dichloromethane (DCM)/trifluoroacetic acid (TFA) mixture as the solvent, and nBu4NBF4 as the electrolyte.
Under these conditions, a heptaphenylene precursor was converted to a hexa-tert-butylhexabenzotriphenylene (pictured above on the right) in a yield of 86 %. Similarly, a quinquephenyl precursor was transformed into a dibenzo[5]helicene in a yield of 51 %. The distorted PAH products showed interesting electronic and optical properties, and the team demonstrated that the reaction is easy to scale up without changing the reaction conditions.
- Electrochemical Continuous-Flow Scholl Reaction toward Polycyclic Aromatic Hydrocarbons,
Wei-Zhen Wang, Qiang Wang, Xinglei He, Yi-Han Shen, Zi’ang Zhai, Ruiying Zhang, Yuanming Li, Ke-Yin Ye,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c00445