Amides and related compounds such as carbamates, ureas, and formamides are important in a wide range of applications, e.g., in medicinal or agrochemistry. The modification of organic molecules with fluorine atoms is often used to tune their properties, for example, for use in drug development. A range of amide-related compounds with an N-CF3 unit can be prepared. Developing methods for similar N-difluoromethylated (N-CF2H) amides could be useful due to the differing properties of CF3 and CF2H groups.
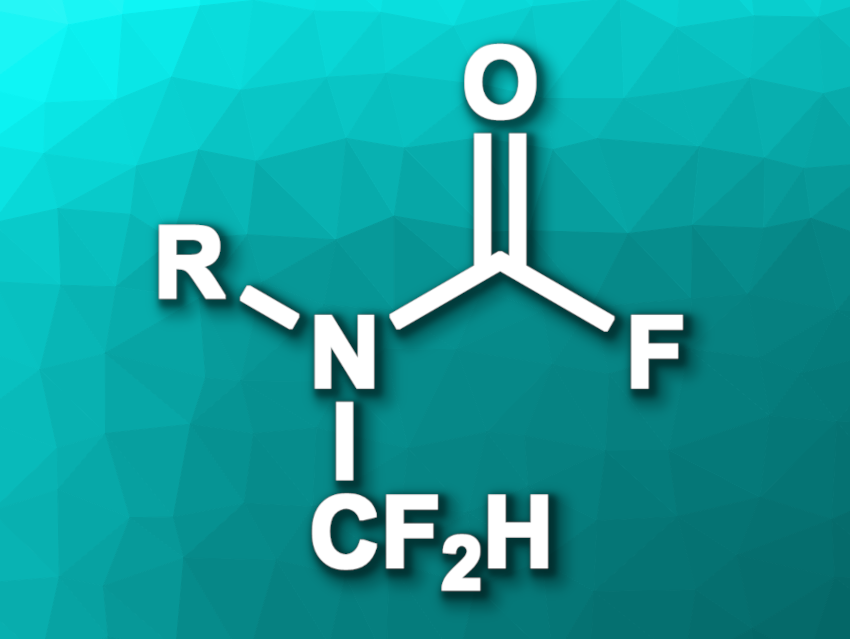
Franziska Schoenebeck, RWTH Aachen University, Germany, and colleagues have developed a method for the synthesis of N-difluoromethyl carbamoyl fluorides (pictured) for the first time, which can serve as building blocks for a variety of previously unexplored N-difluoromethylated compounds. The team prepared N-CF2H carbamoyl fluorides via a desulfurization/fluorination of monosubstituted thioformamides (RNH–CH=S), coupled with a carbonylation using F2C=O formed in situ. They reacted aromatic and aliphatic thioformamides with AgOCF3 and AgF in acetonitrile at 50 °C, with AgOCF3 releasing AgF and F2C=O.
The desired N-difluoromethyl carbamoyl fluorides were obtained in moderate to high yields. The team found that the N-CF2H unit is robust and compatible with a range of different reactions. The N-difluoromethyl carbamoyl fluorides were used as building blocks in further derivatizations to obtain a variety of N-CF2H-functionalized amides, formamides, carbamates, thiocarbamates, and ureas—providing the first efficient access to these target compounds.
- Access to N-Difluoromethyl Amides, (Thio)Carbamates, Ureas, and Formamides,
Filip G. Zivkovic, Gina Wycich, Linhao Liu, Franziska Schoenebeck,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c13711