Sulfur-containing molecules are often used in pharmaceutical chemistry or agrochemistry. Sultines, for example, are cyclic esters of a sulfinic acid, i.e., analogues of lactones and sultones with a tetravalent sulfur atom. Sultines can be found in natural products and bioactive compounds, and they could serve as promising building blocks in drug discovery. However, they have a limited availability in nature and are generally difficult to access synthetically, which hampers their use.
Chao Shu, Central China Normal University, Wuhan, China. and Wuhan Institute of Photochemistry and Technology, China, and colleagues have developed a method that uses photoinduced energy transfer for the construction of polycyclic sultine derivatives. Energy transfer mediated by visible light can be an efficient and environmentally friendly strategy in organic synthesis, for example, for radical cyclization reactions. The team used trifluoromethanesulfinate esters as substrates and induced a radical cyclization under blue light, using 4CzIPN as a cyanoarene-based photocatalyst and Zn(CF3SO2)2 as an additional fluoride source to facilitate the reaction in acetonitrile.
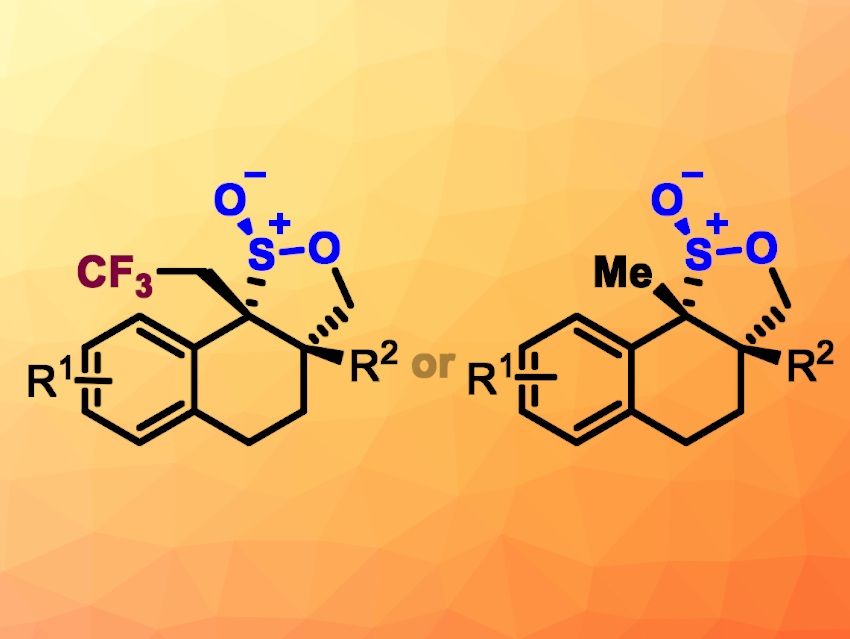
The researchers obtained trifluoromethylated polycyclic sultines (general structure pictured above on the left) in mostly moderate to good yields. Fluorinated compounds such as these can often be particularly useful in pharmaceutical chemistry. Changing the reaction conditions allowed the team to also access alkene hydrosulfination products without the CF3 group (general structure pictured above on the right). Here, they used an iridium-based photocatalyst and tetrahydrofuran (THF) as the solvent.
Overall, the developed cyclization reaction has a good functional group tolerance, proceeds under mild reaction conditions, provides high diastereoselectivity, and is scalable. These properties make it a practical way to access benzofused sultine skeletons, e.g., for drug development.
- Energy‐transfer Enabled Divergent Synthesis of Polycyclic γ‐Sultines,
Pan Zhou, Yongxin Zhang, Xinyue Ma, Xiaoxiao Yang, Xing Fang, Xi Lu, Chao Shu,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202401369