Homocubane is a homolog of cubane in which a methylene group (CH2) is introduced into the cube-shaped parent compound. Azacubanes, i.e., nitrogen-containing analogs of cubanes, are interesting research targets. However, they have turned out to be challenging to synthesize. Similarly, the synthesis of azahomocubanes has proven difficult.
Craig M. Williams, University of Queensland, Brisbane, Australia, and colleagues have performed a synthesis of 1-azahomocubane (pictured above). The team started from commercially available dimethyl 1,4-cubanedicarboxylate, which was converted to aminocubane in seven steps and 69 % overall yield. This intermediate was treated with triflyl azide to obtain cubyl azide. Cubyl azide was converted to a seco-azacubane ester, which was reduced using lithium borohydride to obtain an alcohol intermediate, followed by chlorination using thionyl chloride and treatment with a base. The resulting neutral chloride underwent a cyclization to give the desired 1-azahomocubane.
Overall, 1-azahomocubane was prepared in 17 % yield in 16 steps from dimethyl 1,4-cubanedicarboxylate. The free amine is stable in solution for over a month (at room temperature in the dark). Using density functional theory (DFT) calculations and X-ray diffraction analysis of 1-azahomocubane in a 2:1 complex with silver, the team elucidated the structure of the product (silver complex pictured below).
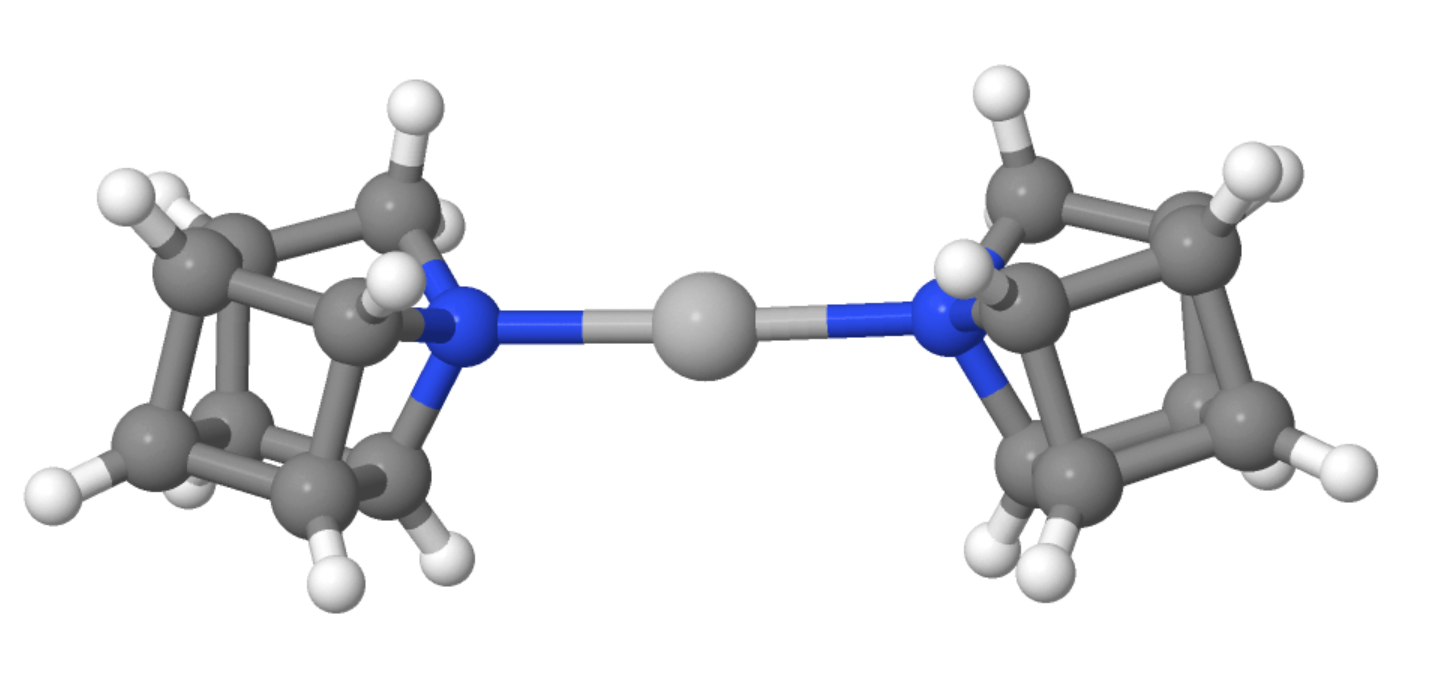
- 1-Azahomocubane,
Tyler Fahrenhorst-Jones, David L. Marshall, Jed. M. Burns, Gregory K. Pierens, Robert E. Hormann, Allison M. Fisher, Paul V. Bernhardt, Stephen J. Blanksby, G. Paul Savage, Philip E. Eaton, Craig M. Williams,
Chem. Sci. 2023.
https://doi.org/10.1039/D3SC00001J




