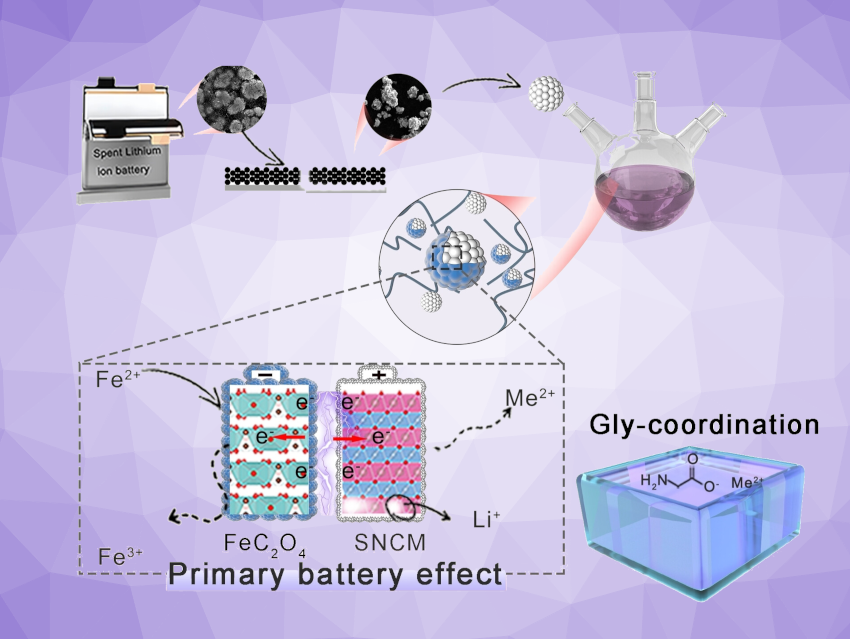
A new strategy for recycling spent lithium-ion batteries is based on a hydrometallurgical process in neutral solution. This process, developed by Lei Ming, Jiexi Wang, Xing Ou, Central South University, Changsha, China, Zhen Yao, Guizhou Normal University, Guiyang, China, and colleagues, allows for the extraction of lithium and other valuable metals in an environmentally friendly, highly efficient, and inexpensive way. The leaching efficiency is improved by a solid-solid reduction mechanism, known as the battery effect, as well as the addition of the amino acid glycine.
Recovering Resources from Spent Lithium-Ion Batteries
Lithium-ion batteries not only power our mobile phones, tablets, and electric vehicles, they are also increasingly important as storage for volatile renewable energy. As they become more widely used, the number of spent batteries keeps increasing. Their recycling is promising, having the potential to reduce environmental impact while extracting raw materials such as lithium, cobalt, nickel, and manganese for the production of new rechargeable batteries.
Current hydrometallurgical methods for the reprocessing of spent lithium-ion batteries are based on acid or ammonia leaching processes. However, excessive and repeated use of acids and bases increases the environmental impact and safety hazards. A pH neutral process would be safer and more environmentally friendly.
Recycling in Neutral Solutions Using Glycine
To come up with a neutral approach, the researchers had to reach deep into their bag of tricks because the aggressive reagents required for classical leaching processes are not easy to replace.
The first “trick”: They constructed “micro batteries” in situ. These help to break up the spent cathode material from the batteries—lithium-coated nickel cobalt manganese oxide (NCM). The NCM particles are mixed with an iron(II) salt, sodium oxalate, and the amino acid glycine in a neutral liquid. This results in the deposition of a thin, solid layer of iron(II) oxalate on the particles. This “shell” acts as an anode while the NCM cores act as the cathode (battery effect). This direct contact allows for easy electron transfer. The coating also hinders the deposition of undesired byproducts on the particles.
The battery effect drives an electrochemical reaction in which the iron(II) ions are oxidized to iron(III) ions and oxygen ions from the oxidic NCM particles are reduced to OH– ions with water. This breaks up the NCM layers, releasing the lithium, nickel, cobalt, and manganese ions they contain into the solution.
Efficient Leaching
In a second “trick”, the released ions are trapped in complexes by the glycine. Glycine also has an additional task: It buffers the pH value of the solution, maintaining a neutral range. Within 15 minutes, it was possible to leach 99.99 % of the lithium, 96.8 % of the nickel, 92.35 % of the cobalt, and 90.59 % of the manganese out of spent cathodes.
This efficient leaching in neutral solution could open new pathways to the realization of large-scale, environmentally friendly recycling of spent batteries. Barely any harmful gases are produced, and the glycine effluent is suitable for use as a fertilizer. This process uses significantly less energy and costs less than conventional methods.
- A Green and Efficient Recycling Strategy for Spent Lithium‐Ion Batteries in Neutral Solution Environment,
Zhilong Xu, Long Ye, Yun Yu, Haiqiang Gong, Zhiming Xiao, Lei Ming, Bao Zhang, Zhen Yao, Jiexi Wang, Xing Ou,
Angew. Chem. Int. Ed. 2025.
https://doi.org/10.1002/anie.202414899