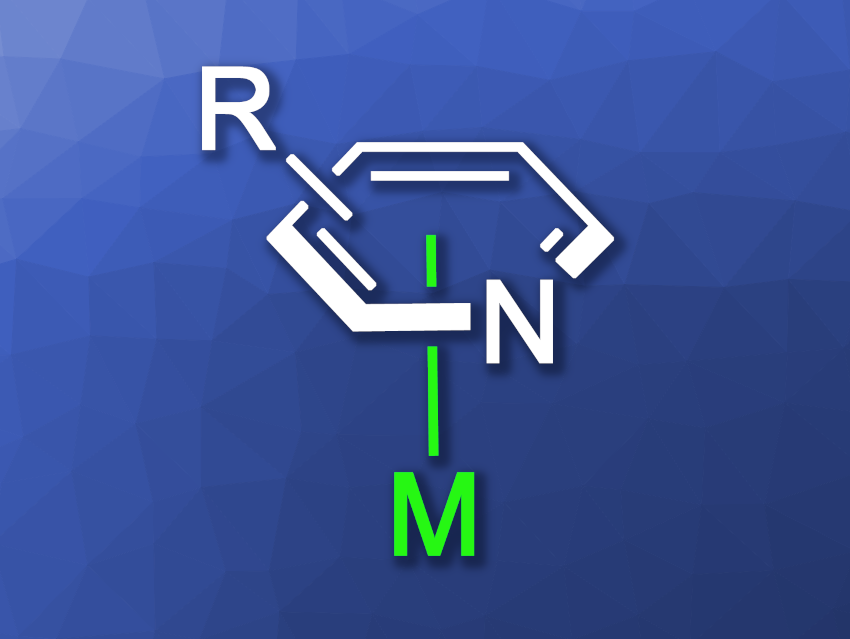
The nitrogen atom in pyridines can coordinate to metals, and the resulting complexes are often useful, e.g., in catalysis. However, this is not the only mode in which pyridine can coordinate to a metal center. The π-electron system can also act as a site for coordination. In an η6-pyridine complex (general structure pictured), for example, the metal is coordinated via all six atoms of the pyridine and sits (close to) centrally over the ring. Activation by such a π-coordination to transition metals has been used to functionalize benzene derivatives. In contrast, the π-coordination of pyridines to transition metals has been less well studied, in part because the coordination to the nitrogen atom in the ring is generally considered more favorable.
Hang Shi, Westlake University and Westlake Institute for Advanced Study, Hangzhou, China, and colleagues have developed a method for aminations of aminopyridines, i.e., substitutions of the NH2 group with other amino groups of the type NHR. The method uses a reversible π-coordination with a ruthenium catalyst to activate the pyridine. The team used the sandwich complex [CptBu3Ru(naphthalene)]NTf2 (CptBu3 = a cyclopentadienyl ligand with three tert-butyl substituents, NTf2 = bistriflimide) as the catalyst for reactions of a range of 2-aminopyridines with a variety of primary amines and cyclic secondary amines in n-heptane at 120 °C.
The desired products were obtained in yields up to 99 %. According to the researchers, the work shows that the activation of pyridines via the π-coordination of transition metals can be achieved in a catalytic way. Thus, it provides a new option for the functionalization of aromatic heterocycles.
- Amination of Aminopyridines via η6-Coordination Catalysis,
Jiajia Chen, Yunzhi Lin, Wen-Qiang Wu, Wei-Qiang Hu, Jingkai Xu, Hang Shi,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c07306