Air-stable organic radicals are challenging to synthesize. Oxygen, in particular, can react easily with radicals. To prevent this, the steric bulk around the radical and the electronic properties of the molecule need to be tuned appropriately. N-heterocyclic carbenes (NHCs), for example, can be useful for stabilizing radical centers. Cyclic (alkyl)(amino)carbenes (cAACs) can serve as useful alternatives to NHCs in some cases.
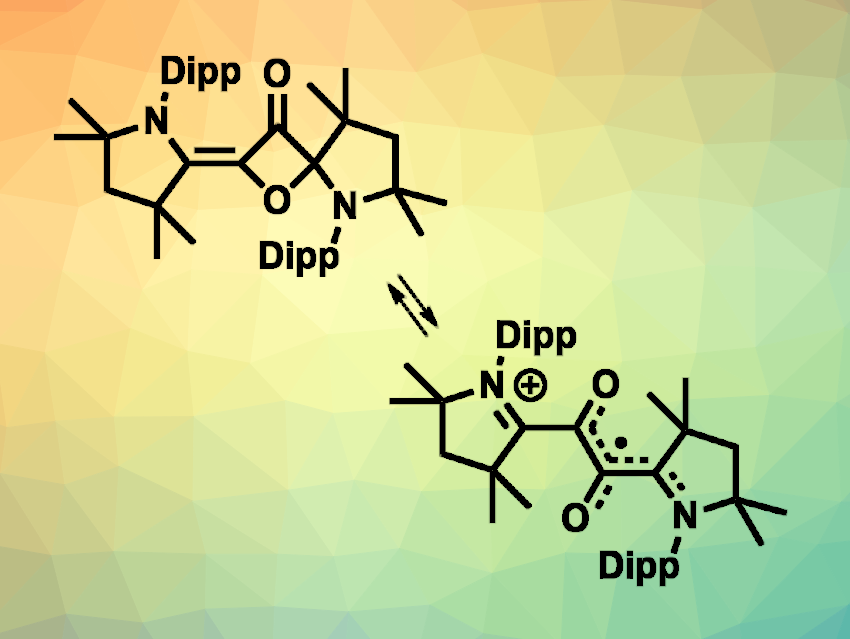
Eunsung Lee, Pohang University of Science and Technology (POSTECH), Republic of Korea, and colleagues have synthesized air-stable organic radicals derived from oxalyl chloride and a cAAC. An initial reaction of oxalyl chloride, i.e., (COCl)2, and a 2,6-diisopropylphenyl (Dipp)-substituted cAAC in the presence of KBF4 in toluene gave a previously known (amino)(carboxy) radical cation and a cAAC-derived 1,2-dicarbonyl radical cation (pictured above on the right). The latter can be reduced completely to a neutral 3-oxetanone derivative (pictured above on the left) when the reaction is performed in tetrahydrofuran (THF) with longer reaction times. The 3-oxetanone derivative and the (amino)(carboxy) radical cation can then be separated.
The isolated 3-oxetanone derivative can be oxidized again using AgBF4 to give the 1,2-dicarbonyl radical cation (and the reaction can be reversed using KCl). The solid-state structure of the cAAC-derived 1,2-dicarbonyl radical cation shows a planar unit that includes the C2O2 group and one pyrrolinium ring, which is involved in the delocalization of the radical. The 1,2-dicarbonyl radical cation is stable in the presence of air and water, with a half-life in wet dichloromethane (DCM) of about 32 days.
- Formation of Highly Stable 1,2-Dicarbonyl Organic Radicals from Cyclic (Alkyl)(amino)carbenes,
Solhye Choe, Hayoung Song, Hyeonjeong Choi, Seunghyuk Yoo, Jaelim Kim, Young Ho Ko, Eunsung Lee,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c01331