Ambiphiles are compounds that behave as both an electrophile and a nucleophile. Stable singlet carbenes, for example, are ambiphiles with both donor and acceptor orbitals at the carbene carbon atom. Electron delocalization can be used to create dual-site ambiphiles with separated donor and acceptor sites. Guy Bertrand, University of California, San Diego, La Jolla, USA, and colleagues have combined both of these concepts.
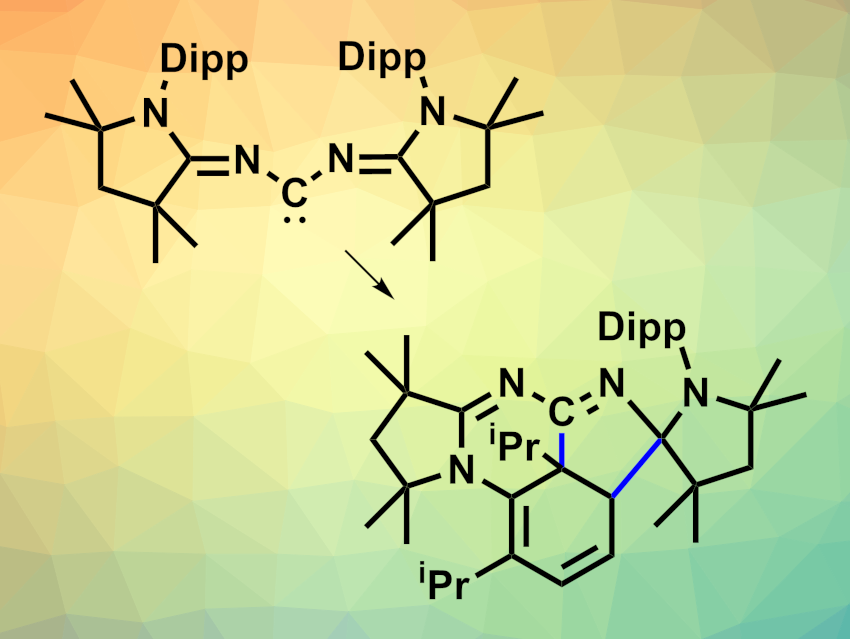
The team synthesized a bis(imino)carbene (pictured above at the top left, Dipp = 2,6-diisopropylphenyl), which has a dual ambiphilic character—both single-site and dual-site—due to delocalization. Due to its unique 1,3-ambiphilic nature, this carbene can undergo a reversible intramolecular dearomative [3 + 2] cycloaddition with one of the Dipp groups. This reaction “masks” the carbene in the form of an air-stable product (pictured above in the bottom right).
The team synthesized the bis(imino)carbene starting from a cyclic (alkyl)(amino)carbene (MeCAAC), which was reacted with bromine to give [MeCAACBr]Br. This intermediate was reacted with formamidine hydrochloride in the presence of triethylamine, followed by LiOTf. The resulting precursor was deprotonated using K[N(SiMe3)2] in tetrahydrofuran (THF) at −78 °C. Above –20 °C, the free carbene is converted to the “masked” form. This process is reversible. According to the researchers, the bis(imino)carbene is the first singlet carbene that can thermally activate an inert arene ring.
- An Air-Stable “Masked” Bis(imino)carbene: A Carbon-Based Dual Ambiphile,
Ying Kai Loh, Mohand Melaimi, Dominik Munz, Guy Bertrand,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.2c12847