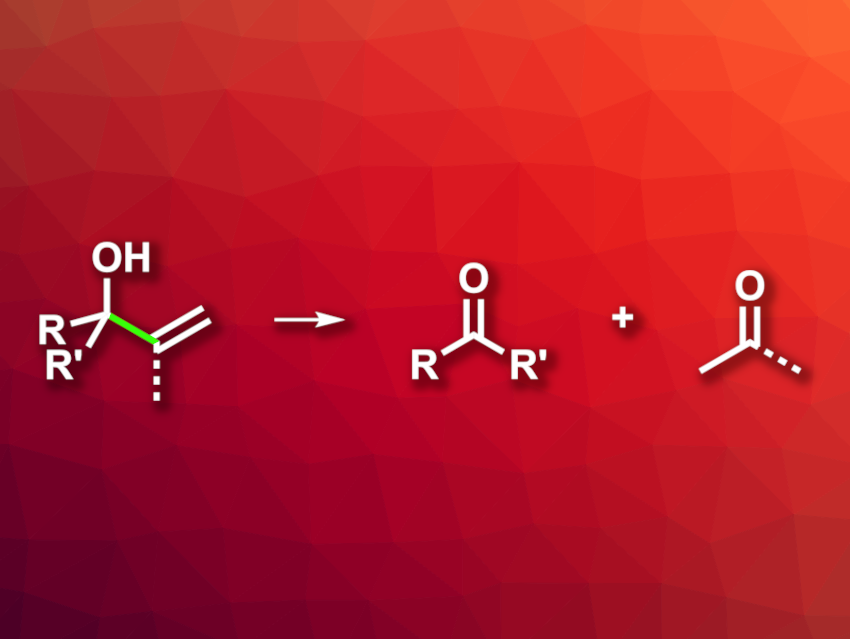
C–C bond cleavage reactions can be useful in organic synthesis. An oxidative C–C bond cleavage in allylic alcohols, for example, can be used to prepare value-added ketones. However, available methods for this type of reaction can suffer from drawbacks such as a need for stoichiometric oxidants or limited substrate scopes.
Meng Deng, Nan Li, Luoyang Normal University, Henan, China, and colleagues have developed a catalytic system for the aerobic C–C bond cleavage of tertiary allylic alcohols to generate ketones (general reaction pictured). The method is based on a hydrogen atom transfer (HAT) process using a CoIII/air/(EtO)3SiH system. The team reacted different allylic alcohols with air in the presence of a CoII(salen) complex as the catalyst together with (EtO)3SiH as a hydrosilane in toluene at 60 °C.
The desired ketones were obtained in high yields and with good chemoselectivity. The team proposes a reaction mechanism that involves the generation of a CoIII–H complex, followed by hydrogen atom transfer to the alkene unit of the allylic alcohol. The resulting alkyl radical reacts with O2, leading to an alkoxy radical. H-abstraction and fragmentation then give the desired ketone. (EtO)3SiH regenerates the catalytically active complex.
- Aerobic C–C Bond Cleavage of Allylic Alcohols via Co-Catalyzed Hydrogen Atom Transfer,
Meng Deng, Mengqi Chu, Nan Li, Guohui Sun, Fei Li, Dade Guo, Guohui Kang, Baoming Ji,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c00556