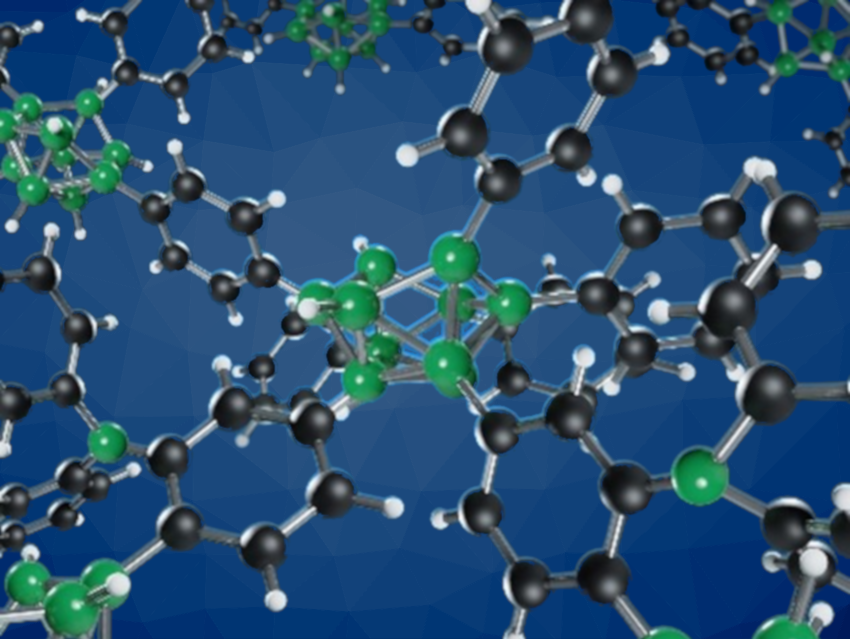
Porous polymers are versatile materials with a broad range of applications. The development of porous materials based on new chemical motifs could open up even more possibilities. For example, so far, borane clusters had not been connected directly by B–B or by short alkyl or aryl bridges to form a porous polymer.
Michael G. S. Londesborough, Jan Demel, Institute of Inorganic Chemistry of the Czech Academy of Sciences, Husinec-Řež, Czech Republic, and colleagues have found that a co-thermolysis of nido-B10H14 and toluene at 250 °C (pictured below) gives a new microporous polymer. The team named the material “activated borane” due to the similarity of its preparation with that of activated carbon.
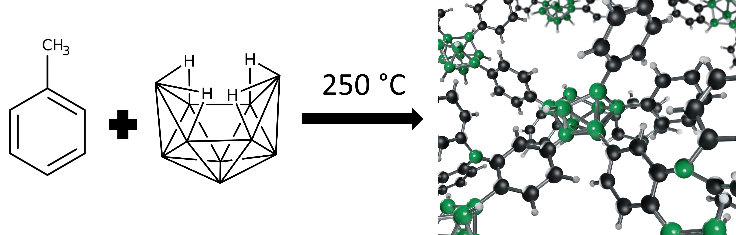
The results of powder X-ray diffraction as well as infrared and NMR spectroscopy suggest that the activated boron has an amorphous structure, featuring borane clusters interconnected via toluene units (pictured above). The product has a high surface area of 774 m2 g–1, shows thermal stability under Ar up to 1000 °C, and has a sorption capacity for pollutants such as sulfamethoxazole, tramadol, diclofenac, and bisphenol A that exceeds the capacity of commercial activated carbon.
The team proposes that activated boron is could be the first member of a new family of porous materials. Preliminary experiments showed that analogous porous polymers can be formed starting from various borane clusters and other simple organic molecules besides toluene. The researchers believe that the potential use of activated boranes could not be limited to the adsorption of pollutants and that future work could reveal other possible applications.
- “Activated Borane” – A Porous Borane Cluster Network as an Effective Adsorbent for Removing Organic Pollutants,
Daniel Bůžek, Karel Škoch, Soňa Ondrušová, Matouš Kloda, Dmytro Bavol, Andrii Mahun, Libor Kobera, Kamil Lang, Michael G. S. Londesborough, Jan Demel,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202201885