The chemistry of s-block metals such as magnesium in low oxidation states can be interesting, e.g., for the development of mild, versatile reducing agents or small-molecule activation. Some low-oxidation-state alkaline-earth-metal complexes can, for example, activate gases such as CO, CO2, or N2. Mg(I) is best explored in this context, with some known examples of reduced Be or Ca complexes. There also are first examples of species with a hetero-bimetallic Mg–Ae bond (Ae = Ca, Sr, Ba) stabilized by an Na–NR2 unit, but an analogous synthesis for the corresponding Be species failed.
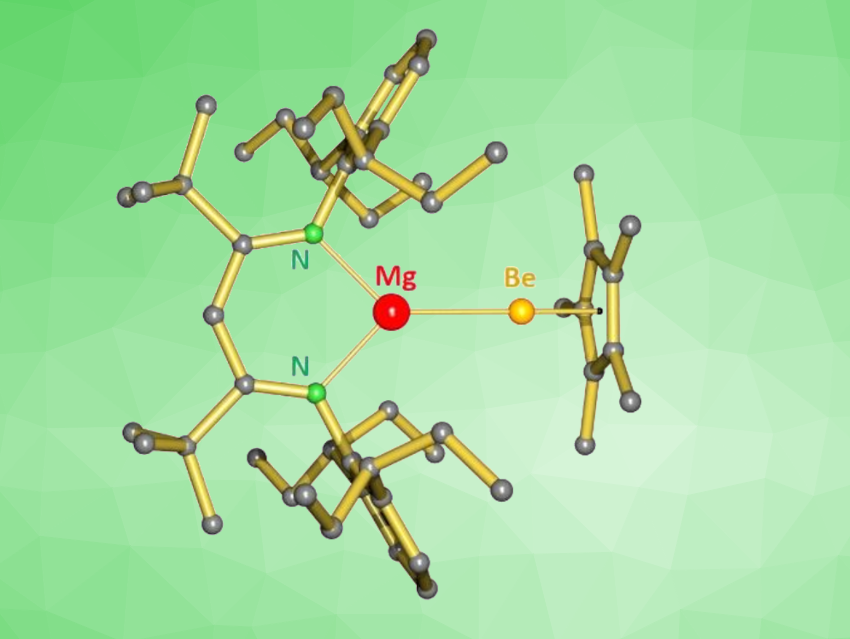
Sjoerd Harder, University Erlangen-Nuremberg, Erlangen, Germany, Magnus R. Buchner, University of Marburg, Germany, and colleagues have synthesized a unique complex with a hetero-bimetallic Mg–Be bond (pictured). The team reacted the dinuclear magnesium complex [(DIPePBDI*)MgNa]2 (DIPePBDI* = HC[(tBu)C=N(DIPeP)]2, DIPeP = 2,6-Et2C-phenyl) with Cp*BeCl (Cp* = pentamethylcyclopentadienyl). They obtained the Mg–Be complex (DIPePBDI*)MgBeCp* in almost quantitative yield in the form of colorless crystals.
The product was characterized using X-ray crystallography and showed a Mg–Be bond length that is close to the sum of the single-bond covalent radii of Mg and Be. The researchers investigated the bonding situation in the complex using density functional theory (DFT) calculation and found that the Mg–Be bond is significantly polarized in a Mgδ+-Beδ– manner. Thus, the product could be described as “beryllyl” complex. It is highly sensitive to air and moisture, but thermally stable. The complex is unreactive towards, e.g., H2, CO, CO2, but can react with some species with C=O, C=S, or N=N bonds.
- Formation, Structure and Reactivity of a Beryllium(0) Complex with Mgδ+ ̶̶ Beδˉ Bond Polarization,
Chantsalmaa Berthold, Johannes Maurer, Lukas Klerner, Sjoerd Harder, Magnus R. Buchner,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202408422